Orientation-controlled BaTiO3 thin films fabricated by chemical solution deposition†
Abstract
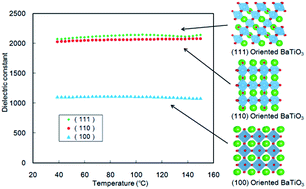
We synthesized orientation-controlled (100), (110), and (111) BaTiO3 films by the chemical solution deposition method. The electric properties of the fabricated BaTiO3 thin films were evaluated, and dielectric constants of 2000 were found for the (110)-oriented and (111)-oriented BaTiO3 film, with temperature dependence stabilized from 20 °C to 150 °C.

 Please wait while we load your content...
Please wait while we load your content...