New Securinega alkaloids with anti-HIV activity from Flueggea virosa†
Hua Zhang‡
a,
Chuan-Rui Zhang‡a,
Ying-Shan Hanb,
Mark A. Wainbergb and
Jian-Min Yue*a
aState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Zhangjiang Hi-Tech Park, Shanghai 201203, P. R. China. E-mail: jmyue@simm.ac.cn; Tel: +86-21-5080-6718
bMcGill University Aids Centre, The Lady Davis Institute for Medical Research, Jewish General Hospital, 3755 Cote Ste-Catherine Road, Montreal, Quebec H3T 1E2, Canada
First published on 3rd December 2015
Abstract
Chemical fractionation of the ethanolic extract of Flueggea virosa yielded a group of Securinega alkaloids including flueggenines E (1) and F (2) as novel hybrid structures, flueggenines G–I (3–5) as new dimers and fluevirosines E–H (6–9) as new trimers, along with six known biosynthetically related compounds. The diverse structures of these isolates were characterized via comprehensive spectroscopic analyses and comparison with literature data. Compounds 1 and 2 are rare Securinega alkaloid hybrids incorporating tryptamine and piperidine residues, respectively, while 3 represents the first example bearing a securinine-type monomeric unit among Securinega alkaloid dimers. An in vitro anti-HIV screening of all available alkaloids revealed weak to moderate activities for over half of these isolates with EC50 values ranging from 7.8 to 122 μM. Among the tested compounds, the known dimer flueggenine D exhibited the best activity with an EC50 of 7.8 ± 0.8 μM.
Introduction
The genus Flueggea Willd. (family Euphorbiaceae) comprises only ca. 12 species but occurs worldwide in both tropical and temperate regions.1 The Flueggea plants are well known in the literature for their diverse secondary metabolites among which the Securinega alkaloids are the most investigated class of compounds.2 F. virosa (Roxb. ex Willd.) Voigt, one of the four Chinese endemic species, is used as folk medicine by local residents for the treatment of eczema and rheumatoid arthritis.1 Previous chemical investigations into the alkaloidal constituents of this herb have yielded all four types of Securinega alkaloids including securinine-type, norsecurinine-type, neosecurinane and neonorsecurinane structures.2–4 Of particular note, F. virosa has so far proven to be the only species to produce Securinega alkaloid oligomers that are all norsecurinine-derived.2Our continuous study of the alkaloids from F. virosa collected at different sites since 2006 has led to the identification of 21 new structures from monomer to pentamer.4–8 It is worthwhile to note that the previous discovery of higher level (n > 2) oligomeric alkaloids was based on a biogenetic proposal6 and relied on a MS-guided separation strategy.7,8 In addition to the above-intended search for routine oligomers, we also noticed some irregular MS peaks indicative of the likely presence of new types of alkaloid oligomers. Further fractionation of the remaining fractions returned two new alkaloid hybrids, flueggenines E (1) and F (2), three new dimers, flueggenines G–I (3–5), and four new trimers, fluevirosines E–H (6–9) (Fig. 1), as well as a known dimer and five known monomers. The new compounds were characterized by spectroscopic means and NMR comparison with known alkaloids. Both new and known compounds were tested in vitro for their anti-HIV effects with more than half showing weak to mild activities. A detailed account of the isolation, structure elucidation and anti-HIV evaluation of these Securinega alkaloids is presented below.
Results and discussion
Positive mode high resolution electrospray ionization mass spectrometry [(+)-HRESIMS] analysis of alkaloid 1 revealed a protonated molecular ion at m/z 378.2193 (calcd 378.2182), supportive of a molecular formula of C23H27N3O2. Inspection of the IR spectrum indicated the existence of an α,β-conjugated lactone moiety (1747 and 1631 cm−1),9 which was further confirmed by the 13C resonances at δC 173.7, 173.5 and 110.3. The NMR data (Tables 1 and 2) for 1 displayed signals for a singlet methyl (δC 39.5; δH 2.56), seven sp3 methylenes (two N-bonded at δC 57.4 & 55.4), three sp3 N-connected methines (δC 66.8, 64.7 & 63.4), five sp2 methines (δH 7.60, 7.37, 7.20, 7.12 & 7.03), four quaternary carbons (a sp3 oxygenated at δC 92.4 and three sp2 ones), and an active proton (δH 8.01). The aforementioned NMR observations were in accord with a 14,15-dihydronorsecurinine fragment6 and a tryptamine residue,9 and this was supported by careful examination of 2D NMR data (Fig. 2). Finally, the connection between the two monomeric units was secured by the diagnostic HMBC correlations from the N-methyl protons to C-15 (δC 64.7) and C-11′ (δC 55.4) and from H2-11′ to C-15, thus defining the planar structure of 1 as shown. The relative configuration of 1 was assigned on the grounds of excellent NMR comparisons (especially proton couplings) with 15β-substituted 14,15-dihydronorsecurinine derivatives,6,10 which was corroborated by subsequent acquisition of ROESY data (Fig. 2) with key correlations of H-2/H-14β and H-15/H-8b. The absolute configuration of 1 was further established via CD data with Cotton effects at 260 (−7.1) and 220 (0.5) nm similar to those observed for a series of 14,15-dihydronorsecurinine derivatives isolated from F. leucopyra.10 Alkaloid 1 was thus identified unambiguously and was named flueggenine E after the known dimers flueggenines A–D5,7 from the same species. A literature search revealed only three examples of Securinega alkaloids oligomerized with other classes of alkaloids: margaritarine, bearing a securinine-type unit and a tryptamine residue, reported in 1991;9 and secu'amamines F and G, incorporating a neosecurinane unit and a piperidine moiety, reported in 2009.11 Flueggenine E (1) represents the fourth Securinega alkaloid hybrid, but the first with a norsecurinine-type monomeric unit.| No. | 1a | 2a | 3a | 4b | 5a |
|---|---|---|---|---|---|
| a Measured in CDCl3.b Measured in CDCl3 with 5% CD3OD.c Interchangeable assignments.d Interchangeable assignments.e Interchangeable assignments.f Interchangeable assignments.g Interchangeable assignments. | |||||
| 2 | 66.8 | 62.8 | 63.2 | 65.1 | 66.2 |
| 3 | 29.3 | 25.9 | 27.0 | 29.3 | 29.26f |
| 4 | 26.8 | 24.8 | 24.3 | 26.82c | 26.8g |
| 5 | 57.4 | 26.8 | 25.6 | 57.8d | 57.3 |
| 6 | 52.62 | 48.9 | |||
| 7 | 63.4 | 57.8 | 58.5 | 64.1 | 63.4 |
| 8 | 33.8 | 66.7 | 42.2 | 31.1 | 30.4 |
| 9 | 92.4 | 36.5 | 89.7 | 92.6 | 90.6 |
| 10 | 84.8 | ||||
| 11 | 173.5 | 173.2 | 172.62e | 173.0 | |
| 12 | 110.3 | 173.4 | 103.6 | 114.7 | 122.9 |
| 13 | 173.7 | 117.4 | 171.8 | 172.8 | 165.4 |
| 14 | 27.4 | 173.6 | 134.7 | 44.2 | 28.1 |
| 15 | 64.7 | 22.7 | 135.4 | 81.5 | 78.7 |
| 2′ | 121.6 | 52.60 | 67.0 | 65.8 | 66.9 |
| 3′ | 114.5 | 28.3 | 29.0 | 29.0 | 29.25f |
| 4′ | 127.6 | 23.2 | 26.6 | 26.76c | 26.9g |
| 5′ | 118.9 | 23.5 | 57.5 | 57.9d | 57.7 |
| 6′ | 119.5 | 46.0 | |||
| 7′ | 122.3 | 65.0 | 64.8 | 64.6 | |
| 8′ | 111.4 | 35.8 | 30.5 | 30.0 | |
| 9′ | 136.4 | 91.9 | 91.6 | 92.5 | |
| 10′ | 22.5 | ||||
| 11′ | 55.4 | 172.6 | 172.4 | 172.8 | |
| 12′ | 110.2 | 111.7 | 110.7 | ||
| 13′ | 172.8 | 172.60e | 174.7 | ||
| 14′ | 29.1 | 27.2 | 26.3 | ||
| 15′ | 42.9 | 41.5 | 39.2 | ||
| OMe | 56.6 | 57.1 | |||
| NMe | 39.5 | ||||
| No. | 1a | 2a | 3a | 4b | 5a |
|---|---|---|---|---|---|
| a Measured in CDCl3.b Measured in CDCl3 with 5% CD3OD. | |||||
| 2 | 3.12 (dd, 8.9, 7.0) | 2.18 (br d, 10.5) | 2.04 (dd, 11.3, 2.6) | 2.96 (dd, 8.8, 7.0) | 3.05 (dd, 8.9, 6.9) |
| 3 | 1.88 (m) | β 1.60 (m) | 1.64 (m) | 1.87 (m) | 1.85–1.94 (m) |
| 1.73 (m) | α 1.38 (m) | 1.54 (m) | 1.77 (m) | 1.71–1.80 (m) | |
| 4 | 1.93 (m) | 1.86 (m) | 1.89 (m) | 1.95 (m) | 1.90–1.98 (m) |
| 1.73 (m) | 1.26 (m) | 1.22 (m) | 1.68 (m) | 1.66–1.76 (m) | |
| 5 | 3.35 (m) | 1.57 (2H, m) | 1.62 (m) | 3.25–3.32 (m) | 3.31 (m) |
| 2.58 (m) | 1.59 (m) | b 2.52–2.63 (m) | 2.62 (m) | ||
| 6 | α 2.80 (m) | 2.98 (m) | |||
| β 2.63 (m) | 2.41 (m) | ||||
| 7 | 3.27 (br d, 6.4) | 2.94 (m) | 3.85 (dd, 5.6, 4.2) | 3.21 (dd, 5.6, 3.9) | 3.13 (m) |
| 8 | a 2.50 (dd, 11.2, 6.4) | 4.13 (dd, 8.7, 5.0) | 2.50 (dd, 9.1, 4.2) | a 2.32 (dd, 11.1, 5.6) | a 2.27 (dd, 11.1, 5.6) |
| b 1.29 (d, 11.2) | 1.72 (d, 9.1) | b 1.75 (d, 11.1) | b 1.70 (d, 11.1) | ||
| 9 | a 2.82 (dd, 13.4, 8.7) | ||||
| b 1.19 (d, 13.4) | |||||
| 12 | 5.63 (d, 2.3) | 5.56 (s) | 5.72 (br s) | ||
| 14 | α 3.03 (m) | 2.61 (d, 12.8) | 2.93 (d, 16.4) | ||
| β 2.69 (ddd, 14.7, 11.1, 2.3) | 2.66 (ddd, 16.4, 5.3, 1.7) | ||||
| 15 | 2.62 (ddd, 11.1, 5.3, 1.5) | α 3.22 (dd, 19.4, 2.4) | 6.67 (d, 5.6) | 3.48 (d, 3.9) | 3.57 (dd, 5.3, 4.1) |
| β 2.76 (dd, 19.4, 2.0) | |||||
| 1′ | 8.01 (br s) | ||||
| 2′ | 7.03 (d, 2.2) | 3.86 (dd, 12.3, 3.1) | 3.10 (dd, 8.8, 7.0) | 3.15 (dd, 9.0, 7.0) | 3.20 (dd, 8.8, 6.8) |
| 3′ | 2.02 (m) | 1.93 (m) | 1.91 (m) | 1.85–1.94 (m) | |
| 1.87 (m) | 1.79 (m) | 1.75 (m) | 1.71–1.80 (m) | ||
| 4′ | 1.98 (m) | 1.96 (m) | 1.93 (m) | 1.90–1.98 (m) | |
| 1.55 (m) | 1.60 (m) | 1.66 (m) | 1.66–1.76 (m) | ||
| 5′ | 7.60 (br d, 7.9) | 1.83 (m) | 3.22 (m) | 3.25–3.32 (m) | 3.39 (m) |
| 1.71 (m) | 2.59 (m) | 2.52–2.63 (m) | 2.64 (m) | ||
| 6′ | 7.12 (ddd, 7.9, 7.2, 0.9) | 3.39 (br d, 12.9) | |||
| 2.92 (ddd, 12.9, 12.3, 3.2) | |||||
| 7′ | 7.20 (ddd, 8.2, 7.2, 1.1) | 3.01 (m) | 2.80 (m) | 3.17 (m) | |
| 8′ | 7.37 (br d, 8.2) | a 2.61 (m) | a 2.42 (dd, 11.3, 5.6) | 2.29 (dd, 11.6, 5.8) | |
| b 1.49 (d, 11.0) | b 1.56 (d, 11.3) | 1.54 (d, 11.6) | |||
| 10′ | 2.95 (2H, m) | ||||
| 11′ | 3.01 (m), 2.97 (m) | ||||
| 12′ | 5.67 (d, 2.1) | 5.72 (br s) | 5.74 (d, 2.4) | ||
| 14′ | 2.96 (m) | 2.91 (m, 2H) | 3.11 (ddd, 17.3, 9.6, 2.4) | ||
| 2.67 (m) | 2.91 (d, 17.3) | ||||
| 15′ | 2.53 (m) | 2.76 (m) | 3.36 (br d, 9.6) | ||
| NMe | 2.56 (s) | ||||
| OMe | 3.30 (s) | 3.20 (s) | |||
Flueggenine F (2) was assigned a molecular formula of C18H26N2O3 based on the (+)-HRESIMS ion at m/z 319.2029 ([M + H]+, calcd 319.2022) indicative of an isomer of secu'amamine F.11 Analysis of the NMR data (Tables 1 and 2) for 2 confirmed this hypothesis with characteristic signals for a neosecurinane substructure and a 2-substituted piperidine moiety. Further examination of 2D NMR data (Fig. 3) established the planar structure of 2 to be identical with that of secu'amamine F, and the critical HMBC correlations from H-2′ (δH 2.56) to C-12 (δC 173.4) and C-13 (δC 117.4) corroborated the linkage between C-2′ and C-13. The relative configuration of the neosecurinane part in 2 was considered to be the same as that of virosine B12 via their highly similar NMR data (particularly the comparable proton couplings) at the corresponding stereocenters (C-2, C-7 and C-8). The ROESY correlations (Fig. 3) of H-2/H-15β, H-3α/H-9a and H-9a/H-8 also supported this assignment. Although the relative configuration at C-2′ remained unassigned due to its distance from the core structure, it was apparent from the coupling patterns (J = 12.3, 3.1 Hz) that H-2′ was axially oriented. The absolute configuration of the neosecurinane part in 2 was determined as shown on the basis of its CD data with Cotton effects at 283 (0.3) and 250 (−0.7) nm.12
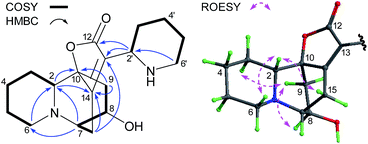
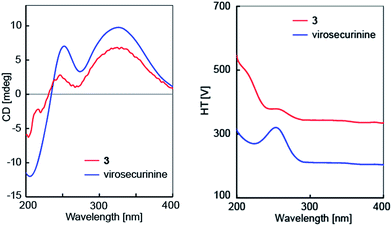
The molecular formula of C25H28N2O4 for flueggenine G (3) was established by HREIMS analysis at m/z 420.2049 (Δmmu 0). The NMR data (Tables 1 and 2) for 3 revealed diagnostic resonances for two γ-carbons (δC 91.9 and 89.7) of the lactones in securinine-/norsecurinine-type alkaloids, suggestive of its dimeric nature. Analysis of 2D NMR data (Fig. 4) established one securinine-type fragment and one dihydronorsecurinine unit that were connected from C-15′ to C-14 via the key HMBC correlations of H-15′/C-13 & C-14. The relative configuration of 3 was assigned on the basis of ROESY data (ESI Fig. S24†) and NMR comparison (ESI Table S1†) with virosecurinine13 and flueggenine A.6 Excellent comparisons between the resonances of monomeric unit A and virosecurinine, and those of monomeric unit B and the dihydronorsecurinine moiety of flueggenine A, supported common configurations at all corresponding chiral centers. In addition, the configuration at the C-15′ stereocenter in 3 was also confirmed by the ROESY correlation of H-15′ (δH 2.53) with H-8′b (δH 1.49). As with the other alkaloid oligomers from the same species,6 alkaloid 3 is likely to be biosynthesized from virosecurinine and (−)-norsecurinine, and thus retains the absolute configurations from the two monomeric precursors. Meanwhile, the CD spectrum (Fig. 5) of 3 showed Cotton effects arising from the α,β,γ,δ-conjugated lactone chromophore comparable to that of virosecurinine, confirmative of the above-mentioned biogenetic correlation. Alkaloid 3 was thereby elucidated to be the first dimeric example derived from a securinine-type monomeric unit.
Flueggenines H (4) and I (5) gave the same molecular formula of C25H30N2O5 as determined from the (+)-HRESIMS ions at m/z 439.2230 and 439.2218 ([M + H]+, calcd 439.2233), respectively. Analysis of the NMR data (Tables 1 and 2) for 4 indicated a MeOH adduct of flueggenine D7 with diagnostic signals for two sp3 methines (δC 44.2 & 81.5; δH 2.61 & 3.48) and a methoxyl group (δC 56.6; δH 3.30) replacing those for Δ14 in the latter. The acquisition of 2D NMR data (Fig. 6) facilitated the aforementioned structural assignment revealing key 1H–1H COSY correlations of H-14/H-15′ and HMBC correlation from the methoxyl protons to C-15 (δC 44.2). The relative configurations at the chiral centers of C-14 and C-15 as shown were assigned via the diagnostic coupling pattern of H-14/H-15 (J14,15 = 0 Hz)8 and the ROESY crosspeaks of OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3/H-8b and H-14/H-8′b. The relative stereostructure of 4 was hence characterized. As with the case of 4 and flueggenine D, the NMR data (Tables 1 and 2) for 5 also suggested it to be a MeOH adduct at Δ14 of flueggenine C,7 and this was supported by the absence of the olefinic resonances and the presence of those for two sp3 methines and one methoxyl group. Examination of 2D NMR data (ESI Fig. Sa†) further confirmed this structural assignment. The relative configuration at the new C-15 stereocenter was suggested by the ROESY correlation of OC
3/H-8b and H-14/H-8′b. The relative stereostructure of 4 was hence characterized. As with the case of 4 and flueggenine D, the NMR data (Tables 1 and 2) for 5 also suggested it to be a MeOH adduct at Δ14 of flueggenine C,7 and this was supported by the absence of the olefinic resonances and the presence of those for two sp3 methines and one methoxyl group. Examination of 2D NMR data (ESI Fig. Sa†) further confirmed this structural assignment. The relative configuration at the new C-15 stereocenter was suggested by the ROESY correlation of OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3/H-8b, while the configurations at the other stereocenters were consistent with their counterparts in flueggenine C7 based on excellent NMR comparisons and ROESY data (ESI Fig. S42†).
3/H-8b, while the configurations at the other stereocenters were consistent with their counterparts in flueggenine C7 based on excellent NMR comparisons and ROESY data (ESI Fig. S42†).
The molecular formula of C37H44N3O7 for 6 was determined through 13C NMR data and the (+)-HRESIMS ion at m/z 642.3173 ([M + H]+, calcd 642.3179). Analysis of the NMR data (Tables 3 and 4) for 6 established that it was a trimer derived from 5 by the addition of a dihydronorsecurinine unit at C-12′. Indeed, the absence of the H-12′ signal and the presence of those for a dihydronorsecurinine residue were supportive of this structural variation, which was further confirmed by 2D NMR data (ESI Fig. Sa†) with corroborative HMBC correlations from H-15′′ (δH 3.30) to C-11′ (δC 172.4), C-12′ (δC 121.2) and C-13′ (δC 168.6). The new C-15′′ chiral center was considered to possess identical relative configuration with C-15′ based on the shielded C-8′′ signal (δC 30.2),6,7 while those at all other stereocenters were assigned as drawn via NMR comparison with 5 and analysis of ROESY data (ESI Fig. S51†). The structure of 6 was hence elucidated and was named fluevirosine E after the known trimers fluevirosines A–D.6,7
| No. | 6 | 7 | 8 | 9 |
|---|---|---|---|---|
| a Interchangeable assignments.b Interchangeable assignments.c Interchangeable assignments.d Interchangeable assignments.e Interchangeable assignments.f Interchangeable assignments.g Interchangeable assignments.h Interchangeable assignments.i Interchangeable assignments.j Interchangeable assignments.k Interchangeable assignments. | ||||
| 2 | 66.5a | 65.1 | 65.1 | 65.5j |
| 3 | 29.3b | 29.3e | 29.5g | 29.5 |
| 4 | 26.9c | 26.88f | 27.0h | 27.0k |
| 5 | 57.55d | 54.8 | 55.2 | 55.3 |
| 7 | 63.3 | 58.8 | 59.5 | 59.4 |
| 8 | 30.8 | 36.4 | 36.3 | 35.7 |
| 9 | 90.4 | 91.4 | 92.3 | 92.2 |
| 11 | 173.4 | 172.1 | 172.34i | 172.1 |
| 12 | 122.6 | 120.6 | 107.4 | 107.0 |
| 13 | 166.8 | 162.9 | 169.3 | 169.4 |
| 14 | 27.9 | 132.8 | 133.2 | 134.9 |
| 15 | 78.6 | 139.9 | 139.2 | 141.1 |
| 2′ | 67.1 | 66.5 | 67.5 | 65.1 |
| 3′ | 29.21b | 29.22e | 29.4g | 29.2 |
| 4′ | 26.84c | 26.88f | 26.93h | 26.8k |
| 5′ | 57.52d | 57.8 | 57.8 | 58.1 |
| 7′ | 66.0 | 67.5 | 64.3 | 65.4j |
| 8′ | 30.4 | 30.4 | 35.8 | 31.5 |
| 9′ | 90.4 | 91.8 | 90.7 | 92.9 |
| 11′ | 172.4 | 172.8 | 173.9 | 171.8 |
| 12′ | 121.2 | 109.8 | 122.3 | 113.9 |
| 13′ | 168.6 | 173.5 | 166.1 | 173.2 |
| 14′ | 25.1 | 26.76f | 26.9h | 42.9 |
| 15′ | 37.9 | 38.8 | 43.1 | 47.7 |
| 2′′ | 66.4a | 67.1 | 67.2 | 66.0 |
| 3′′ | 29.16b | 29.18e | 29.2g | 29.1 |
| 4′′ | 26.80c | 26.86f | 26.7h | 26.8k |
| 5′′ | 57.46d | 58.1 | 57.5 | 57.9 |
| 7′′ | 65.5 | 65.7 | 65.2 | 64.8 |
| 8′′ | 30.2 | 36.0 | 35.7 | 30.2 |
| 9′′ | 92.1 | 91.7 | 91.9 | 91.6 |
| 11′′ | 172.6 | 173.4 | 173.0 | 172.5 |
| 12′′ | 110.0 | 110.9 | 110.6 | 112.0 |
| 13′′ | 174.0 | 172.3 | 172.31i | 171.9 |
| 14′′ | 25.8 | 28.4 | 27.8 | 27.4 |
| 15′′ | 38.5 | 42.9 | 39.3 | 44.3 |
| OMe | 57.0 | |||
| No. | 6 | 7 | 8 | 9 |
|---|---|---|---|---|
| 2 | 3.20 (dd, 8.9, 6.8) | 3.06 (m) | 3.15 (8.9, 7.1) | 3.07 (m) |
| 3 | 1.81–1.96 (m) | 1.89–1.99 (m) | 2.01 (m) | 1.99 (m) |
| 1.66–1.80 (m) | 1.72–1.82 (m) | 1.73–1.83 (m) | 1.70–1.83 (m) | |
| 4 | 1.90–2.02 (m) | 1.92–2.01 (m) | 1.92–2.03 (m) | 1.92–2.02 (m) |
| 1.64–1.78 (m) | 1.68–1.79 (m) | 1.63–1.77 (m) | 1.66–1.79 (m) | |
| 5 | 3.28–3.40 (m) | 3.26 (m) | 3.32 (m) | 3.22 (m) |
| 2.55–2.69 (m) | 2.52 (m) | 2.54 (m) | 2.49 (m) | |
| 7 | 3.17 (m) | 3.66 (dd, 6.5, 4.5) | 3.70 (dd, 6.5, 4.5) | 3.61 (dd, 6.5, 4.6) |
| 8 | 2.28 (m) | 2.54 (dd, 10.6, 4.5) | 2.60 (dd, 10.5, 4.5) | 2.54 (dd, 10.6, 4.6) |
| 1.72 (d, 11.0) | 1.64 (d, 10.6) | 1.74 (d, 10.5) | 1.57 (d, 10.6) | |
| 12 | 5.72 (s) | 5.82 (s) | ||
| 14 | 2.76 (d, 16.6) | |||
| 2.70 (dd, 16.6, 5.1) | ||||
| 15 | 3.61 (dd, 5.1, 4.2) | 6.92 (d, 6.5) | 6.73 (d, 6.5) | 6.26 (d, 6.5) |
| 2′ | 3.15 (m) | 3.17 (dd, 8.9, 6.9) | 3.12 (m) | 3.11 (dd, 8.9, 6.7) |
| 3′ | 1.81–1.96 (m) | 1.89–1.99 (m) | 1.87–1.95 (m) | 1.88–1.97 (m) |
| 1.66–1.80 (m) | 1.72–1.82 (m) | 1.73–1.83 (m) | 1.70–1.83 (m) | |
| 4′ | 1.90–2.02 (m) | 1.92–2.01 (m) | 1.92–2.03 (m) | 1.92–2.02 (m) |
| 1.64–1.78 (m) | 1.68–1.79 (m) | 1.63–1.77 (m) | 1.66–1.79 (m) | |
| 5′ | 3.28–3.40 (m) | 3.30 (m) | 3.24 (m) | 3.37 (m) |
| 2.55–2.69 (m) | 2.53 (m) | 2.52 (m) | 2.54 (m) | |
| 7′ | 2.94 (m) | 2.85 (dd, 5.5, 2.5) | 3.22 (brd, 5.9) | 2.97 (d, 5.4) |
| 8′ | 2.26 (dd, 11.5, 5.8) | 2.32 (dd, 11.3, 5.5) | 2.62 (dd, 11.1, 5.9) | 2.32 (dd, 11.4, 5.4) |
| 1.63 (d, 11.5) | 2.17 (d, 11.3) | 1.47 (d, 11.1) | 1.52 (d, 11.4) | |
| 12′ | 5.66 (br s) | 5.69 (s) | ||
| 14′ | 2.95 (m, 2H) | 2.99 (m, 2H) | 3.56 (dd, 16.0, 5.0) | 2.78 (d, 10.1) |
| 2.75 (dd, 16.0, 12.4) | ||||
| 15′ | 3.34 (m) | 3.42 (m) | 2.51 (m) | 2.98 (s) |
| 2′′ | 3.01 (m) | 3.14 (dd, 9.0, 7.0) | 3.10 (dd, 9.2, 6.9) | 3.15 (dd, 8.9, 6.9) |
| 3′′ | 1.81–1.96 (m) | 1.89–1.99 (m) | 1.87–1.95 (m) | 1.88–1.97 (m) |
| 1.66–1.80 (m) | 1.72–1.82 (m) | 1.73–1.83 (m) | 1.70–1.83 (m) | |
| 4′′ | 1.90–2.02 (m) | 1.92–2.01 (m) | 1.92–2.03 (m) | 1.92–2.02 (m) |
| 1.64–1.78 (m) | 1.68–1.79 (m) | 1.63–1.77 (m) | 1.66–1.79 (m) | |
| 5′′ | 3.28–3.40 (m) | 3.27 (m) | 3.13 (m) | 3.30 (m) |
| 2.55–2.69 (m) | 2.54 (m) | 2.58 (m) | 2.59 (m) | |
| 7′′ | 2.96 (m) | 3.00 (m) | 3.01 (m) | 2.82 (m) |
| 8′′ | 2.30 (dd, 11.3, 6.3) | 2.64 (dd, 10.9, 5.9) | 2.55 (dd, 11.1, 6.1) | 2.40 (dd, 11.2, 5.7) |
| 1.88 (d, 11.3) | 1.56 (d, 10.9) | 1.53 (d, 11.1) | 1.55 (d, 11.2) | |
| 12′′ | 5.67 (d, 2.1) | 5.72 (d, 2.0) | 5.66 (d, 2.0) | 5.59 (d, 1.5) |
| 14′′ | 3.11 (d, 16.0) | 2.86 (m) | 2.99 (dd, 14.7, 5.2) | 2.94 (m) |
| 3.02 (m) | 2.78 (ddd, 15.9, 12.9, 2.0) | 2.81 (ddd, 14.7, 12.1, 2.0) | 2.59 (m) | |
| 15′′ | 3.30 (m) | 2.90 (m) | 2.88 (m) | 2.94 (m) |
| OMe | 3.26 (s) |
Fluevirosines F (7) and G (8) exhibited quasi-molecular ion peaks in the (+)-HRESIMS spectra at m/z 610.2934 and 610.2910 ([M + H]+), respectively, corresponding to the same molecular formula of C36H39N3O6 and indicative of a pair of isomers. The NMR data (Tables 3 and 4) for 7 were highly similar to those for fluevirosine D7 with the only difference being attributable to signals around C-15′′, which suggested 7 to be a likely 15′′-epimer of the latter. Analysis of 2D NMR data (ESI Fig. Sa†) for 7 confirmed that they possess the same planar structure bearing one norsecurinine and two dihydronorsecurine moieties. Compared to fluevirosine D,7 the coupling pattern of H2-14′′ with H-15′′, the chemical shift (δC 36.0) for C-8′′ and the ROESY correlation of H-8′′b/H-15′′ all supported an inverted configuration at C-15′′. The relative configurations at all other stereocenters were determined to be the same as those in fluevirosine D by their excellent NMR comparisons and analysis of ROESY data (ESI Fig. S60†). Similar to the case of 7 and fluevirosine D, alkaloid 8 was determined to be the 15′′-epimer of fluevirosine A6 by analysis of the NMR data (Tables 3 and 4). The relative configuration at C-15′′ was established as described for 7, and inspection of 2D NMR data (1H–1H COSY, HMBC and ROESY, ESI Fig. Sa & S69†) further corroborated the aforementioned assignment. The structures of 7 and 8 were thus characterized.
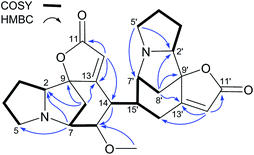
Fluevirosine H (9) gave a molecular formula (C36H39N3O6) same as 8 based on the (+)-HRESIMS ion at m/z 610.2924 ([M + H]+, calcd 610.2917). Compared to 8, the NMR data (Tables 3 and 4) for 9 also exhibited the presence of one norsecurinine and two dihydronorsecurinine fragments with extra signals for a olefinic proton (δH 5.69, H-12′) and a methine group (δH 2.78 & δC 42.9, CH-14′) but absence of those for a methylene and a quaternary carbon, which suggested a distinct oligomerization pattern. Further analysis of 2D NMR data (Fig. 7) confirmed the presence of the aforementioned monomeric substructures and the connections between them with a confirmatory 1H–1H COSY correlation of H-14′/H-15′′ and HMBC correlations from H-15′ (δH 2.98) to C-13 (δC 169.4), C-14 (δC 134.9), C-15 (δC 141.4) and C-15′′ (δC 44.3). The relative configurations at C-14′, C-15′ and C-15′′ were established to be identical with those at the corresponding chiral centers in fluevirosinine E8 via the same coupling patterns of H-14′, H-15′ and H-15′′, while the other stereocenters were assigned on the basis of excellent NMR comparisons with the above-described alkaloids. The absolute configurations of 7–9 were determined to be as shown by analysis of their CD spectra, which displayed Cotton effects at 270 (−10.5), 270 (−14.6) and 267 (−13.2) nm, respectively.8
In addition to the above-mentioned new alkaloids, six known ones, flueggeainol,14 bubbialine,15 (+)-14,15-dihydronorsecurine,16 14,15-epoxynorsecurinine,17 15α-methoxy-14,15-dihydronorsecurine,10 and flueggine B,18 were also obtained and identified by full spectroscopic analyses and comparison with reported data in the literature.
All available Securinega alkaloids from F. virosa, including those (virosecurinine,6 viroallosecurinine,6 (−)-norsecurinine,6 flueggenines C and D,7 and fluevirosine D7) reported previously, were tested for their in vitro anti-HIV activities on HIV-1 NL 4-3 infected MT4 cells and nevirapine was used as the positive control.19 The assay results (Table 5) revealed that more than half of these compounds showed mild protection on MT-4 cells against the HIV-induced cytopathic effect (EC50 10–100 μM) without displaying cytotoxicity (CC50 > 100 μM). The best anti-HIV activity was observed for a known dimer, flueggenine D, with an EC50 of 7.8 ± 0.8 μM (selective index = 12.6).
| Compds | EC50 | CC50 |
|---|---|---|
| Flueggenine E (1) | 42.6 ± 4.3 | >100 |
| Flueggenine F (2) | — | — |
| Flueggenine G (3) | — | — |
| Flueggenine H (4) | 122 ± 12 | — |
| Flueggenine I (5) | 63.9 ± 6.5 | — |
| Fluevirosine E (6) | — | — |
| Fluevirosine F (7) | — | — |
| Fluevirosine G (8) | 58.7 ± 5.6 | — |
| Fluevirosine H (9) | 108 ± 10 | — |
| Flueggeainol | 41.9 ± 4.2 | >100 |
| Bubbialine | — | — |
| 14,15-Dihydronorsecurine | — | — |
| 14,15-Epoxynorsecurinine | 85.5 ± 8.7 | — |
| 15α-Methoxy-14,15-dihydronorsecurine | 89.0 ± 9.2 | — |
| Flueggine B | 79.6 ± 8.1 | — |
| Flueggenine C | — | — |
| Flueggenine D | 7.8 ± 0.8 | 97.9 |
| Fluevirosine D | — | — |
| Virosecurinine | 19.3 ± 2.0 | >100 |
| Viroallosecurinine | 56.4 ± 5.7 | — |
| (−)-Norsecurinine | 43.0 ± 4.4 | >100 |
| Nevirapine | 0.119 ± 0.012 | >100 |
Experimental
General experimental procedures
Optical rotations were obtained on a Rudolph Autopol VI automatic polarimeter. UV and IR spectra were acquired on a Shimadzu UV-2550 UV/Visible spectrophotometer and a Perkin-Elmer 577 spectrometer, respectively. NMR experiments were performed on a Bruker AM-500 spectrometer with a cryoprobe referenced to deuterated solvent peaks (δH 7.26 and δC 77.23 for CDCl3). LR- and HR-EIMS analyses (70 eV) were performed on a Finnigan MAT 95 mass spectrometer. LR- and HR-ESIMS experiments were conducted on Bruker Daltonics esquire3000plus and Waters LCT Premier XE spectrometers, respectively. Pre-coated silica gel GF254 plates (Yantai Huiyou Silica Gel Exploitation Company, Ltd., China) were used for TLC analyses and separations. Silica gel H (300–400 mesh, Qingdao Haiyang Chemical Plant, Ltd., China), Sephadex LH-20 gel (Pharmacia Biotech, Sweden), and amino silica gel (20–45 μm, Fuji Silysia Chemical, Ltd., Japan) were used for column chromatography (CC). HPLC purifications were carried out on a Waters 1525 binary pump system equipped with a 2489 UV/Visible detector and a XBridge Prep C18 column (5 μm, 10 × 250 mm). All solvents used for CC were of at least analytical grade (Shanghai Chemical Reagents Company, Ltd., China), and solvents used for UV, [α]D, and NMR measurements were of suitable chromatographic grades from Merck or Sigma-Aldrich.Plant materials
The plant materials (twigs and leaves) of F. virosa were collected in April 2007 from Xishuangbanna of Yunnan Province and in September 2007 from Gongchen of Guangxi Zhuang Autonomous Region, respectively. They were authenticated by Prof. Y. K. Xu from Xishuangbanna Tropical Botanical Garden and by Prof. S. Q. Tang from Guangxi Normal University, respectively. The voucher specimens have been deposited in the herbarium of Shanghai Institute of Materia Medica (Accession numbers: 2007-FV-1Y and 2007-FV-2Y, respectively).Extraction and isolation
The crude alkaloids (11.1 g) of the Xishuangbanna species were obtained as previously reported.5 Fractionation of the crude alkaloids with a MCI gel column (MeOH–H2O, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 7
7 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) yielded four major fractions F1–F4. After crystallization of virosecurinine from F3, the mother solution was subjected to silica gel CC (CHCl3–CH3OH, 500
3) yielded four major fractions F1–F4. After crystallization of virosecurinine from F3, the mother solution was subjected to silica gel CC (CHCl3–CH3OH, 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to furnish ten sub-fractions, the fourth of which was further purified by Sephadex LH-20 CC (in MeOH) to give flueggenine G (3, 3.0 mg).
1) to furnish ten sub-fractions, the fourth of which was further purified by Sephadex LH-20 CC (in MeOH) to give flueggenine G (3, 3.0 mg).
A total of 28.2 g crude alkaloids were prepared from the Gongchen species through the same procedures as described formerly.7 Fractionation of the crude alkaloids with a silica gel column (petroleum ether–EtOAc–HNEt2, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 1
0.1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2) returned six major fractions F1–F6, and F4 (7.76 g) was further subjected to silica gel CC (CHCl3–MeOH, 100
0.2) returned six major fractions F1–F6, and F4 (7.76 g) was further subjected to silica gel CC (CHCl3–MeOH, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford subfractions F4a–F4g. Subfraction F4b was sequentially separated by silica gel CC (petroleum ether–EtOAc–HNEt2, 5
1) to afford subfractions F4a–F4g. Subfraction F4b was sequentially separated by silica gel CC (petroleum ether–EtOAc–HNEt2, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 1.5
0.1 to 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), amino silica gel CC (petroleum ether–EtOAc, 1
0.1), amino silica gel CC (petroleum ether–EtOAc, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and preparative TLC (petroleum ether–EtOAc–HNEt2, 1.5
2), and preparative TLC (petroleum ether–EtOAc–HNEt2, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2) to give 14,15-epoxynorsecurinine (6.6 mg) and 15α-methoxy-14,15-dihydronorsecurine (8.5 mg). Subfraction F4d was processed with amino silica gel (CHCl3–MeOH, 50
0.2) to give 14,15-epoxynorsecurinine (6.6 mg) and 15α-methoxy-14,15-dihydronorsecurine (8.5 mg). Subfraction F4d was processed with amino silica gel (CHCl3–MeOH, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to furnish three elutions, and 4 (1.9 mg) and (+)-14,15-dihydronorsecurine (4.5 mg) were purified from the first and third elutions by HPLC with MeCN–H2O system (20–80% and 20–40%, respectively, over 20 min). Subfraction F4e was fractionated by HPLC with MeCN–H2O system (30–45% over 20 min) to yield 5 (5.6 mg) and a mixture which was further separated by HPLC with MeOH–H2O as mobile phase (35–60% over 20 min) to give 9 (2.5 mg) and 7 (3.7 mg).
1) to furnish three elutions, and 4 (1.9 mg) and (+)-14,15-dihydronorsecurine (4.5 mg) were purified from the first and third elutions by HPLC with MeCN–H2O system (20–80% and 20–40%, respectively, over 20 min). Subfraction F4e was fractionated by HPLC with MeCN–H2O system (30–45% over 20 min) to yield 5 (5.6 mg) and a mixture which was further separated by HPLC with MeOH–H2O as mobile phase (35–60% over 20 min) to give 9 (2.5 mg) and 7 (3.7 mg).
Fraction F5 (3.27 g) was first separated over silica gel (CHCl3–MeOH, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford subfractions F5a–F51, and six more elutions (F5a1–F5a6) were further obtained by subsequent fractionation of F5a (2.06 g) on a silica gel column (petroleum ether–EtOAc–HNEt2, 4
1) to afford subfractions F5a–F51, and six more elutions (F5a1–F5a6) were further obtained by subsequent fractionation of F5a (2.06 g) on a silica gel column (petroleum ether–EtOAc–HNEt2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 2
0.1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1). Flueggeainol (1.8 mg) was obtained from F5a1 by HPLC purification (30–60% MeCN–H2O over 15 min), while bubbialine (9.6 mg) and 1 (2.3 mg) were acquired from F5a6 also by HPLC separation (MeCN–H2O, 25–30% over 15 min, to 50% in 0.5 min then to 80% over 6 min). Subfraction F5c was further fractionated by HPLC (25–60% MeCN–H2O over 20 min) to furnish 8 (1.9 mg), while subfractions F5g and F5i yielded flueggine B (1.9 mg) and 2 (2.7 mg) via HPLC purifications eluted with gradient (25–60% over 20 min) and isocratic (37%) MeCN–H2O, respectively. All solvent systems used for HPLC separations were at a flow rate of 3.5 mL min−1 and modified with 0.02% HNEt2 unless specified.
0.1). Flueggeainol (1.8 mg) was obtained from F5a1 by HPLC purification (30–60% MeCN–H2O over 15 min), while bubbialine (9.6 mg) and 1 (2.3 mg) were acquired from F5a6 also by HPLC separation (MeCN–H2O, 25–30% over 15 min, to 50% in 0.5 min then to 80% over 6 min). Subfraction F5c was further fractionated by HPLC (25–60% MeCN–H2O over 20 min) to furnish 8 (1.9 mg), while subfractions F5g and F5i yielded flueggine B (1.9 mg) and 2 (2.7 mg) via HPLC purifications eluted with gradient (25–60% over 20 min) and isocratic (37%) MeCN–H2O, respectively. All solvent systems used for HPLC separations were at a flow rate of 3.5 mL min−1 and modified with 0.02% HNEt2 unless specified.
Characterization of new compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 260 (3.93), 220 (4.46) nm; CD (MeOH) λ (Δε) 260 (−7.1), 220 (0.5), 210 (2.1) nm; IR (KBr) νmax 2964, 1747, 1631, 1599, 1564, 1552, 1381, 1354, 1221, 1075, 911, 744 cm−1; 1H and 13C NMR data see Tables 1 and 2; ESIMS m/z 378.2 [M + H]+, 376.1 [M − H]−; (+)-HRESIMS m/z 378.2193 [M + H]+ (calcd for C23H28N3O2, 378.2182).
ε) 260 (3.93), 220 (4.46) nm; CD (MeOH) λ (Δε) 260 (−7.1), 220 (0.5), 210 (2.1) nm; IR (KBr) νmax 2964, 1747, 1631, 1599, 1564, 1552, 1381, 1354, 1221, 1075, 911, 744 cm−1; 1H and 13C NMR data see Tables 1 and 2; ESIMS m/z 378.2 [M + H]+, 376.1 [M − H]−; (+)-HRESIMS m/z 378.2193 [M + H]+ (calcd for C23H28N3O2, 378.2182).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 219 (4.05) nm; CD (MeOH) λ (Δε) 283 (0.3), 250 (−0.7), 223 (8.5) nm; IR (KBr) νmax 2941, 2850, 1745, 1670, 1442, 1414, 1344, 1201, 1174, 1124, 1080, 1014 cm−1; 1H and 13C NMR data see Tables 1 and 2; ESIMS m/z 319.2 [M + H]+, 637.2 [2M + H]+; (+)-HRESIMS m/z 319.2029 [M + H]+ (calcd for C18H27N2O3, 319.2022).
ε) 219 (4.05) nm; CD (MeOH) λ (Δε) 283 (0.3), 250 (−0.7), 223 (8.5) nm; IR (KBr) νmax 2941, 2850, 1745, 1670, 1442, 1414, 1344, 1201, 1174, 1124, 1080, 1014 cm−1; 1H and 13C NMR data see Tables 1 and 2; ESIMS m/z 319.2 [M + H]+, 637.2 [2M + H]+; (+)-HRESIMS m/z 319.2029 [M + H]+ (calcd for C18H27N2O3, 319.2022).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 257 (3.74), 212 (4.10) nm; CD (MeOH) λ (Δε) 324 (3.5), 247 (1.5) nm; IR (KBr) νmax 3431 (br, H2O), 2926, 2852, 1755, 1649, 1618, 1259, 1076 cm−1; 1H and 13C NMR data see Tables 1 and 2; EIMS (70 eV) m/z 420 ([M]+, 12), 337 (19), 191 (36), 149 (14), 84 (100); HREIMS m/z 420.2049 [M]+ (calcd for C25H28N2O4, 420.2049).
ε) 257 (3.74), 212 (4.10) nm; CD (MeOH) λ (Δε) 324 (3.5), 247 (1.5) nm; IR (KBr) νmax 3431 (br, H2O), 2926, 2852, 1755, 1649, 1618, 1259, 1076 cm−1; 1H and 13C NMR data see Tables 1 and 2; EIMS (70 eV) m/z 420 ([M]+, 12), 337 (19), 191 (36), 149 (14), 84 (100); HREIMS m/z 420.2049 [M]+ (calcd for C25H28N2O4, 420.2049).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 215 (4.14) nm; IR (KBr) νmax 2960, 2927, 1758, 1641, 1465, 1385, 1227, 1144, 1080, 975, 918 cm−1; 1H and 13C NMR data see Tables 1 and 2; ESIMS m/z 439.3 [M + H]+, 877.4 [2M + H]+; (+)-HRESIMS m/z 439.2230 [M + H]+ (calcd for C25H31N2O5, 439.2233).
ε) 215 (4.14) nm; IR (KBr) νmax 2960, 2927, 1758, 1641, 1465, 1385, 1227, 1144, 1080, 975, 918 cm−1; 1H and 13C NMR data see Tables 1 and 2; ESIMS m/z 439.3 [M + H]+, 877.4 [2M + H]+; (+)-HRESIMS m/z 439.2230 [M + H]+ (calcd for C25H31N2O5, 439.2233).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 216 (4.41) nm; IR (KBr) νmax 2963, 2874, 1754, 1670, 1643, 1460, 1420, 1292, 1233, 1220, 1097, 1081, 1065, 919, 784 cm−1; 1H and 13C NMR data see Tables 1 and 2; ESIMS m/z 439.4 [M + H]+, 877.6 [2M + H]+; (+)-HRESIMS m/z 439.2218 [M + H]+ (calcd for C25H31N2O5, 439.2233).
ε) 216 (4.41) nm; IR (KBr) νmax 2963, 2874, 1754, 1670, 1643, 1460, 1420, 1292, 1233, 1220, 1097, 1081, 1065, 919, 784 cm−1; 1H and 13C NMR data see Tables 1 and 2; ESIMS m/z 439.4 [M + H]+, 877.6 [2M + H]+; (+)-HRESIMS m/z 439.2218 [M + H]+ (calcd for C25H31N2O5, 439.2233).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 224 (4.33) nm; IR (KBr) νmax 2960, 2928, 1752, 1645, 1458, 1421, 1291, 1232, 1114, 1067, 998, 918 cm−1; 1H and 13C NMR data see Tables 3 and 4; ESIMS m/z 642.4 [M + H]+, 1283.8 [2M + H]+; (+)-HRESIMS m/z 642.3173 [M + H]+ (calcd for C37H44N3O7, 642.3179).
ε) 224 (4.33) nm; IR (KBr) νmax 2960, 2928, 1752, 1645, 1458, 1421, 1291, 1232, 1114, 1067, 998, 918 cm−1; 1H and 13C NMR data see Tables 3 and 4; ESIMS m/z 642.4 [M + H]+, 1283.8 [2M + H]+; (+)-HRESIMS m/z 642.3173 [M + H]+ (calcd for C37H44N3O7, 642.3179).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 270 (3.99), 214 (4.26) nm; CD (MeOH) λ (Δε) 270 (−10.5) nm; IR (KBr) νmax 2958, 2875, 1757, 1645, 1616, 1460, 1418, 1262, 1223, 1198, 1144, 1117, 1077, 978, 917 cm−1; 1H and 13C NMR data see Tables 3 and 4; ESIMS m/z 305.7 [M + 2H]2+, 610.4 [M + H]+, 1219.6 [2M + H]+; (+)-HRESIMS m/z 610.2934 [M + H]+ (calcd for C36H40N3O6, 610.2917).
ε) 270 (3.99), 214 (4.26) nm; CD (MeOH) λ (Δε) 270 (−10.5) nm; IR (KBr) νmax 2958, 2875, 1757, 1645, 1616, 1460, 1418, 1262, 1223, 1198, 1144, 1117, 1077, 978, 917 cm−1; 1H and 13C NMR data see Tables 3 and 4; ESIMS m/z 305.7 [M + 2H]2+, 610.4 [M + H]+, 1219.6 [2M + H]+; (+)-HRESIMS m/z 610.2934 [M + H]+ (calcd for C36H40N3O6, 610.2917).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 259 (3.99), 217 (4.39) nm; CD (MeOH) λ (Δε) 270 (−14.6) nm; IR (KBr) νmax 2961, 2872, 1755, 1646, 1622, 1458, 1284, 1243, 1222, 1144, 1111, 1079, 973, 915 cm−1; 1H and 13C NMR data see Tables 3 and 4; ESIMS m/z 305.8 [M + 2H]2+, 610.4 [M + H]+; (+)-HRESIMS m/z 610.2910 [M + H]+ (calcd for C36H40N3O6, 610.2917).
ε) 259 (3.99), 217 (4.39) nm; CD (MeOH) λ (Δε) 270 (−14.6) nm; IR (KBr) νmax 2961, 2872, 1755, 1646, 1622, 1458, 1284, 1243, 1222, 1144, 1111, 1079, 973, 915 cm−1; 1H and 13C NMR data see Tables 3 and 4; ESIMS m/z 305.8 [M + 2H]2+, 610.4 [M + H]+; (+)-HRESIMS m/z 610.2910 [M + H]+ (calcd for C36H40N3O6, 610.2917).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 260 (4.04), 216 (4.31) nm; CD (MeOH) λ (Δε) 267 (−13.2) nm; IR (KBr) νmax 2961, 2874, 1756, 1644, 1622, 1484, 1458, 1286, 1248, 1231, 1143, 1113, 1077, 966, 918, 854 cm−1; 1H and 13C NMR data see Tables 3 and 4; ESIMS m/z 305.8 [M + 2H]2+, 610.4 [M + H]+, 1219.6 [2M + H]+; (+)-HRESIMS m/z 610.2924 [M + H]+ (calcd for C36H40N3O6, 610.2917).
ε) 260 (4.04), 216 (4.31) nm; CD (MeOH) λ (Δε) 267 (−13.2) nm; IR (KBr) νmax 2961, 2874, 1756, 1644, 1622, 1484, 1458, 1286, 1248, 1231, 1143, 1113, 1077, 966, 918, 854 cm−1; 1H and 13C NMR data see Tables 3 and 4; ESIMS m/z 305.8 [M + 2H]2+, 610.4 [M + H]+, 1219.6 [2M + H]+; (+)-HRESIMS m/z 610.2924 [M + H]+ (calcd for C36H40N3O6, 610.2917).Bioassays
The anti-HIV and cytotoxic activities of the tested alkaloids on MT-4 cell cultures were measured as described previously19 with minor modifications. Briefly, MT-4 cells were added to 96-well plates containing serial dilutions of the tested compounds. HIV-1 NL 4-3 was used to infect the MT-4 cells at a final multiplicity of infection (MOI) of 0.03. The assay plates were incubated in a humidified incubator at 37 °C under 5% CO2. After 3 or 4 days, 10 mL of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (5 mg mL−1 in PBS) was added into each well and the plates were incubated for another 3 h at 37 °C. A lysis buffer (10% (v/v) Triton X-100 in acidified isopropanol) was added to each well to lyse the cells and to solubilize the formazan crystal. The plates were read at a wavelength of 570 nm on a FLUOStar Optima plate reader (BMG Labtech). EC50 (50% effective concentration) is defined as the concentration of each compound achieving 50% protection on MT-4 cells against the HIV-induced cytopathic effect, and CC50 (50% cytotoxic concentration) in parallel is defined as the concentration of each compound killing 50% of the MT-4 cells. Both values were determined using Prism 5.0 software (GraphPad, San Diego, CA).Acknowledgements
This project was financially supported by the National Natural Science Foundation (No. 81322045, 21532007). We thank Prof. S. Q. Tang of Guangxi Normal University for the collection and identification of the plant material.Notes and references
- B. Li, M. G. Gilbert, G. Fischer and C. A. Meyer, Flora of China, Science Press, Missouri Botanical Garden Press, Beijing, St. Louis, 2008, vol. 11, p. 178 Search PubMed.
- E. Chirkin, W. Atkatlian and F.-H. Porée, The Alkaloids: Chemistry and Biology, Academic Press, Pittsburgh, 2015, vol. 74, pp. 1–120 Search PubMed.
- X. H. Li, M. M. Cao, Y. Zhang, S. L. Li, Y. T. Di and X. J. Hao, Tetrahedron Lett., 2014, 55, 6101–6104 CrossRef CAS.
- L. S. Gan and J. M. Yue, Nat. Prod. Commun., 2006, 1, 819–823 CAS.
- L. S. Gan, C. Q. Fan, S. P. Yang, Y. Wu, L. P. Lin, J. Ding and J. M. Yue, Org. Lett., 2006, 8, 2285–2288 CrossRef CAS PubMed.
- H. Zhang, C. R. Zhang, K. K. Zhu, A. H. Gao, C. Luo, J. Li and J. M. Yue, Org. Lett., 2013, 15, 120–123 CrossRef CAS PubMed.
- H. Zhang, W. Wei and J. M. Yue, Tetrahedron, 2013, 69, 3942–3946 CrossRef CAS.
- H. Zhang, Y. S. Han, M. A. Wainberg and J. M. Yue, Tetrahedron, 2015, 71, 3671–3679 CrossRef CAS.
- D. Arbain, A. A. Birkbeck, L. T. Byrne, M. V. Sargent, B. W. Skelton and A. H. White, J. Chem. Soc., Perkin Trans. 1, 1991, 1863–1869 RSC.
- G. Wang, Y. Wang, X. Zhang, Y. I. X. Yao and W. Ye, Chem. Pharm. Bull., 2010, 58, 390–393 CrossRef CAS PubMed.
- A. Ohsaki, T. Nagaoka, K. Yoneda and A. Kishida, Tetrahedron Lett., 2009, 50, 6965–6967 CrossRef CAS.
- G. C. Wang, Y. Wang, Q. Li, J. P. Liang, X. Q. Zhang, X. S. Yao and W. C. Ye, Helv. Chim. Acta, 2008, 91, 1124–1129 CrossRef CAS.
- H. Wu and J. Zhou, Zhongguo Zhongyao Zazhi, 2004, 29, 535–537 CAS.
- M. Chen and L. Hou, Zhiwu Xuebao, 1985, 27, 625–629 CAS.
- A. Ahond, J. Guilhem, J. Hamon, J. Hurtado, C. Poupat, J. Pusset, M. Pusset, T. Sevenet and P. Potier, J. Nat. Prod., 1990, 53, 875–881 CrossRef CAS.
- G. Han, M. G. LaPorte, J. J. Folmer, K. M. Werner and S. M. Weinreb, J. Org. Chem., 2000, 65, 6293–6306 CrossRef CAS PubMed.
- E. V. Dehmlow, M. Guntenhoner and T. van Ree, Phytochemistry, 1999, 52, 1715–1716 CrossRef CAS.
- B. X. Zhao, Y. Wang, D. M. Zhang, R. W. Jiang, G. C. Wang, J. M. Shi, X. J. Huang, W. M. Chen, C. T. Che and W. C. Ye, Org. Lett., 2011, 13, 3888–3891 CrossRef CAS PubMed.
- C. Pannecouque, D. Daelemans and E. de Clercq, Nat. Protoc., 2008, 3, 427–434 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Full spectroscopic data including 1D & 2D NMR, IR and MS spectra for all new compounds. See DOI: 10.1039/c5ra22191a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |