Investigating the stability of gold nanorods modified with thiol molecules for biosensing†
Abstract
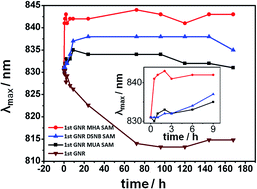
Gold nanorods (GNRs) modified with functional molecules are useful in chemical and biological sensing. In this work, the extinction spectrum of purified GNRs prepared by seed-mediated growth is systematically characterized by UV-visible absorption spectroscopy to test its stability before and after alkane-thiol modification. The results show that purification of GNRs makes the GNRs less stable, while a GNRs colloid solution functionalized by 11-mercaptoundecanoic acid (MUA), 3,3′-dithiobis[6-nitrobenzoic acid]bis(succinimide)ester (DSNB), or 16-mercaptohexadecanoic acid (MHA), respectively is stable over time. Bovine serum albumin (BSA) as a sample protein can be successfully attached to DSNB modified GNRs and form stable BSA–DSNB-GNRs probes. The red-shift observed in the extinction spectrum due to the BSA attachment is consistent over repeated experiments. Finally, anti-PSA (prostate-specific antigen) as a capture antibody is also attached to DSNB-modified GNRs. The attached anti-PSA is capable of interacting with prostate-specific antigens and induces a further red-shift, suggesting potential applications of thiol modified GNRs in bio-sensing.

 Please wait while we load your content...
Please wait while we load your content...