Ultra-small Au nanoparticles stabilized by silica hollow nanospheres for styrene oxidation with oxygen
Yuanyuan Zheng†
ab,
Xiaoming Zhang†bc,
Yi Yaobc,
Xiaohong Chen*a and
Qihua Yang*b
aXihua University, Chengdu 310014, China
bState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023, China. E-mail: yangqh@dicp.ac.cn
cGraduate School of the Chinese Academy of Sciences, Beijing 100049, China
First published on 7th December 2015
Abstract
Ultra-small Au nanoparticles (<2 nm) supported on hollow silica nanospheres were successfully fabricated with the aid of chemical modification (–SH groups) or through simple immersion method, leading to supported catalysts Au/SH-HNS and Au/HNS. The resulting solid catalysts showed good thermal stability and the small particle sizes could be remained even at high temperature of 350 °C. Such catalysts were catalytically active in the oxidation of styrene using O2 as oxidant under 1 atm pressure. The catalytic results showed that the activity strongly depends on the Au loading amount, and the loading of 4–5 wt% led to the highest activity. Significant rate enhancement was observed with gold nanoparticles supported on pure silica in comparison with thiol modified silica nanospheres, suggesting the negative effects of thiol groups. The solid catalyst could be reused at least 8 reaction cycles without significant decrease in activity and selectivity. This study not only supplies an active, recoverable catalyst for the green transformation of styrene, but also demonstrates that the hollow silica nanosphere material has a superior ability in stabilizing metal nanoparticles against growth.
1. Introduction
The catalytic aerobic transformation of alkenes is of great interest and importance in the production of fine chemicals and chemical intermediates. The process is generally performed using organic or inorganic oxidants, such as tert-butyl hydroperoxide, or KMnO4, which might produce environmentally undesirable wastes.1–4 Molecular oxygen, as a green oxidant with obvious advantages including atom economy and low cost, has attracted considerable attentions.5,6 However, the use of molecular oxygen in alkenes oxidations is still a challenge.Recently, an increasing interest has been directed to the catalytic potential of gold catalysts in olefin oxidations since the pioneering work of Haruta who reported the remarkable catalytic activity of supported Au nanoparticles in directly oxidation of propene using molecular oxygen.7 The particle size of Au NPs is a critical factor for determining the catalytic performance, and ultra-small Au nanoparticle catalysts (<5 nm) have attracted considerable attentions due to their unique geometrical and electronic structures, making them promising candidates for novel catalytic sites.8,9 However, because of the rapid decreasing of melting point when Au particle size is reduced into nanoscale and Ostwald ripening, these particles are prone to agglomeration and sintering, leading to drastic loss of catalytic activity. Thus, stabilizing these ultra-small Au nanoparticles to enhance their resistance to sintering and deactivation is highly desirable.
Different strategies have been employed to enhance the catalytic activity and impede nanoparticle sintering under high temperature.10–16 One important rout is to incorporate Au nanoparticles in the framework of supports.17–19 For example, Song and co-workers demonstrated a nanoreactor catalyst with superb thermal stability by embedding highly dispersed Au nanoparticles into the inner wall of TiO2 hollow spheres.17 Wan and co-workers reported a coordination-assisted synthetic approach for the synthesis of highly active and stable gold nanoparticle catalysts in ordered mesoporous carbon materials.20 Encapsulation of the dispersed gold nanoparticles inside porous silica, CeO2, zeolite, etc. has been proven another important route to prevent nanoparticle sintering.14,21–23 For example, Mou and co-workers encapsulated monodispersed small Au nanoparticles inside hollow nanospheres through a water-in-oil microemulsion method.21 The presence of protection shell is feasible for enhancing the thermal stability against sintering. Though these strategies mentioned above have been proved to be efficient, developing simple method is still highly desirable.
It is proved that the support structure and pore architecture play a key role in stabilizing metal nanoparticles.24,25 Among various supports, our recently synthesized silica hollow nanospheres may be a good candidate for preventing the aggregation or the growth of metal nanoparticles due to the rather unique structure.26 Different to the widely used channel-like mesoporous materials such as MCM-41 and SBA-15, this material possess a small particle size less than 20 nm and microwindows on the shell. The isolated nanocaves of the hollow nanospheres can not only accommodate metal particles and provide specific microenvironments for catalytic reactions but also prevent the undesired aggregation and agglomeration of the metal nanoparticles. However, such support has not been explored to support Au nanoparticles as far as we know.
In this article, we report the construction of hollow silica nanosphere as efficient support for loading ultrasmall Au nanoparticles. The relationship of the Au loading, particle size and catalytic activity in the styrene oxidation using O2 as oxidant was investigated. This study not only leads to an active catalyst for the oxidation of styrene but also demonstrates the superior potentials of the hollow silica nanosphere material for preventing the aggregation or growth of metal nanoparticles.
2. Results and discussion
2.1 Characterization of silica hollow nanospheres before and after Au loading
The hollow silica nanosphere functionalized with thiol group was synthesized using F127 as a single micelle template under neutral conditions. FT-IR spectra of silica hollow nanospheres are summarized in Fig. 1(A). All the samples exhibit the characteristic bands from silica at around 3450, 1640 and 1095 cm−1 respectively for ν(O–H), δ(O–H), and ω(Si–O). In comparison with pure silica samples, two additional peaks corresponding to the stretching and bending vibrations of C–H appeared at 2940 and 1450 cm−1 in HS-HNS, which demonstrate the successful introduction of –SH groups in the hollow nanospheres. This is further demonstrated by Raman spectra. As shown in Fig. 1(B), compared with HNS, SH-HNS exhibits additional peaks attributing to S–H group at around 2577 cm−1. The amount of –SH groups was 1.72 mmol g−1 based on S element analysis. After reduction with H2, no obvious changes could be found in FT-IR spectrum of Au/SH-HNS, indicating the existence of –SH groups after reduction. After thermal treatment under air atmosphere at 350 °C, the vibration for C–H disappeared, demonstrating that thiol groups were removed by air treatment.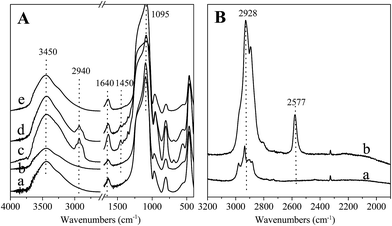 | ||
| Fig. 1 (A) FT-IR spectra of (a) HNS, (b) Au/HNS, (c) SH-HNS, (d) Au/SH-HNS, (e) Au/SH-HNS-air; (B) Raman spectra of (a) HNS, (b) SH-HNS. | ||
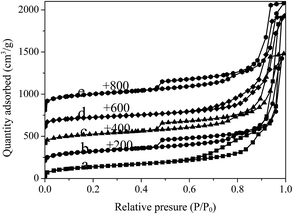
The porosity of silica hollow nanospheres before and after Au loading was characterized by N2 sorption measurement (Fig. 2). All the samples exhibit two capillary condensation steps in the N2 isotherm pattern, indicating that two types of pores coexist in the materials. The first hysteresis loop at relative pressure of 0.50–0.90 is from the void space of the hollow interior, and the second capillary condensation at relative pressure of 0.90–1.0 is from the void space of the aggregated nanospheres. The BET surface area of silica hollow nanospheres (HNS) and thiol functionalized silica hollow nanospheres (SH-HNS) are calculated to be 502 and 455 m2 g−1 respectively (Table 1). After loading with Au nanoparticles, slightly decrease in pore volume were observed, probably due to nanopore occupation by Au NPs. It should be mentioned that Au/SH-HNS-air exhibits higher surface area and pore volume than Au/SH-HNS (714 vs. 466 m2 g−1, 1.98 vs. 1.67 cm3 g−1) attributing to the remove of organic groups.
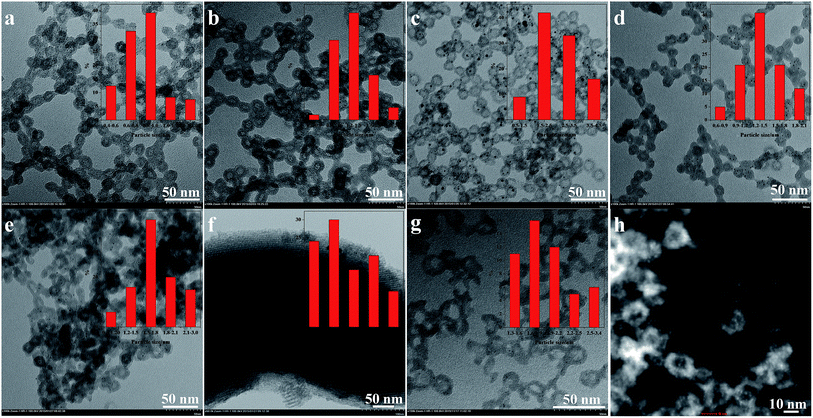
Transmission electron microscopic (TEM) images of silica hollow nanospheres loaded with Au are presented in Fig. 3. The Au/SH-HNS catalysts clearly show the existence of monodispersed hollow nanosphere with diameter of 18 ± 2 nm, wall thickness of 3.5 ± 0.5 nm and hollow cavity of 10–14 nm. As coordination agent, the presence of –SH groups was a critical factor for the adsorption of HAuCl4 from solution. The high adsorption capacity of SH-HNS allowed us to adjust the loadings of Au in a wide range, and the loading amount could be easily controlled between 0.57–6.81 wt% by adjusting the amount of added metal source (Table 2). The TEM images of representative catalysts Au/SH-HNS with Au loading of 4.40 wt% and 6.81 wt% are shown in Fig. 3a and b. The TEM images clearly show the uniform distribution of Au nanoparticles with a narrow size distribution inside the hollow nanocaves of silica hollow nanospheres. For the Au loading of 4.40 wt%, the particle sizes of Au is in a range of 1.0 ± 0.20 nm. When the Au loading was increased up to 6.81 wt%, the particle size increases slightly to 1.4 ± 0.25 nm. For better examine the detailed morphology of the hybrid Au catalysts, high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image of Au/SH-HNS (4.40 wt%) was taken (Fig. 3h). As clearly seen, the particle size of Au is in a range of 1.0–1.3 nm and most of them uniformly distributed inside the hollow nanocaves of silica nanospheres. After –SH groups were removed by calcination at 350 °C in air, the nanoparticle size of Au increases from 1 ± 0.20 nm to about 3.2 ± 0.84 nm (Fig. 3c, Table 2). The growth of Au nanoparticles might be attributed to the high treatment temperature and the removal of –SH groups. Notably, Au/SH-HNS exhibited excellent high-temperature durability. After thermal treatment under 350 °C (H2 atmosphere), the particle size could be still kept below 1.5 nm (Fig. 3d). Encouragingly, the particle size of Au prepared by simple impregnation method was also smaller than 1.9 ± 0.34 nm using the silica hollow nanosphere as support (Fig. 3e). In comparison, the particle size of Au is larger than 8 nm with SBA-15 using similar impregnation method (Fig. 3f). These results demonstrate that the unique nanostructure of silica hollow nanosphere support is beneficial for obtaining ultra-small Au nanoparticles, probably due to the spatial restriction of the limited space of hollow nanosphere and the microwindows in the shell.
| Entry | Catalyst | Au loadingb (wt%) | Average particle size (nm) | Conv. (%) | Sel. (%) | ||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| a Reaction conditions: 5.5 mmol of styrene, 1.5 μmol of Au for each catalyst, 3 mL of dioxane, 100 °C, 12 h, 1 atm O2.b The Au loading content was detected by ICP measurement.c The reaction time is 11 h.d The reaction time is 9 h. | |||||||
| 1 | Au/SH-HNS | 0.57 | <1.0 | 18.6 | 8.3 | 84.2 | 7.5 |
| 2 | Au/SH-HNS | 1.15 | <1.0 | 30.1 | 27.6 | 61.6 | 10.8 |
| 3 | Au/SH-HNS | 2.06 | <1.0 | 31.8 | 40.6 | 47.6 | 11.8 |
| 4 | Au/SH-HNS | 4.40 | 1.0 ± 0.20 | 37.0 | 57.3 | 32.9 | 9.8 |
| 5 | Au/SH-HNS | 6.81 | 1.4 ± 0.25 | 27.7 | 53.8 | 39.3 | 6.9 |
| 6 | Au/SH-HNS-air | 5.76 | 3.2 ± 0.84 | 44.3 | 57.7 | 33.8 | 8.5 |
| 7 | Au/HNSc | 4.0 | 1.9 ± 0.34 | 45.6 | 68.6 | 23.2 | 8.2 |
| 8 | Au/SBA-15d | 4.0 | 8.4 ± 2.63 | 41.4 | 68.1 | 22.2 | 9.7 |
2.2 The oxidation of styrene with oxygen as oxidant
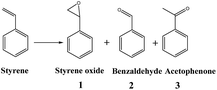
The catalytic performance of the solid catalysts was evaluated for styrene epoxidation using molecular oxygen as oxidant, and the results are summarized in Table 2. In order to achieve the highest activity, a set of catalysts Au/SH-HNS with Au loading varying from 0.57 to 1.15, 2.06, 4.40 and 6.81 wt% were investigated (entries 1–5). Mainly three products are detected as listed in Table 2. With low loading content of 0.57 wt%, Au/HS-HNS exhibits poor activity with conversion of 18.6%. As the loading content increases, the activity of Au/HS-HNS increases significantly. The maximum styrene conversion of 37.0% was obtained with the Au loading of 4.40 wt%. Further increasing the loading content to 6.81 wt%, the decrease in activity could be observed, and only 27.7% styrene conversion was obtained under the same reaction conditions. The particle size only changes in the range of <1.0 nm to 1.4 nm with Au loading in the range of 0.57 to 6.81 wt%. As we will show later the styrene oxidation is not size-sensitive with Au particle size less than 10 nm. The low activity at high loading is probably mainly due to the crowded active sites, which will not be beneficial for the mass transportation. The selectivity to styrene epoxide follows the similar tendency to the activity and reaches the maximum on Au/SH-HNS with Au loading of 4.40 wt%. With higher conversion, the selectivity to styrene epoxide is better and the selectivity to benzaldehyde will be lower. However, the product selectivity tendency of Au/SH-HNS with high loading of 6.81 wt% is different. Its selectivity to styrene epoxide is much higher than 1.15 wt% catalyst while the selectivity to benzaldehyde is much lower though its conversion is a little lower. This phenomenon might be attributed to the crowded active sites that is beneficial for the over oxidation of substrate to benzoic acid or other products, leading to much lower benzaldehyde and acetophenone selectivity. Since –SH groups show strong interaction with Au metals, which might influence the catalytic activity, a catalyst without thiol groups was also prepared by removing –SH under air atmosphere using Au/SH-HNS-air with 5.76 wt% Au loading as a model catalyst. Due to the thiol removal, slightly increase in Au loading from 4.40 to 5.76 wt% was observed after calcination. Au/SH-HNS-air only shows slightly higher styrene conversion than Au/SH-HNS (44.3% vs. 37.0%). The selectivity to styrene epoxide remains almost the same for Au/SH-HNS before and after thiol removal. This suggests the thiol removal could increase the catalyst activity of Au nanoparticles, probably due to the increase in the number of exposed active sites.Notably, the activity and selectivity to styrene oxide of Au/HNS (4.0 wt%) are much higher than those of Au/SH-HNS and Au/SH-HNS-air (entry 7). Within 11 h, the conversion could reach as high as 45.6% with selectivity to styrene epoxide of 68.6%. This suggests that thiol may have negative effect on the catalytic performance of Au in the styrene oxidation reaction. Under similar reaction conditions, Au/SBA-15 with larger Au particle size exhibits good activity and selectivity towards styrene oxidation with a conversion of 41.4% and selectivity of 68.1% towards styrene oxidate within 9 h (entry 8). This indicates that styrene oxidation is not size sensitive with the size of less than 10 nm.
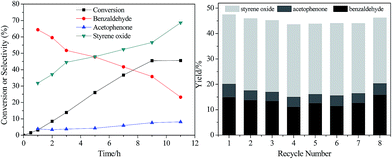
The time course plots for styrene oxide are shown in Fig. 4a with Au/HNS as a model catalyst. From the time course plots, we can see that the conversion increase linearly with the reaction time within 9 h. The zero-order reaction curve indicates a strong adsorption of styrene on the Au NPs, showing the free diffusion of reactants during the catalytic process. However, almost no increase in styrene conversion was observed after 9 h. During the reaction process, the selectivity changed a lot. At the initial stage, we can see that benzaldehyde was the main product and it decreases sharply with the reaction time. On the contrary, the selectivity to styrene oxide increases greatly with the reaction time increasing. Styrene oxide becomes the main products at high conversion, with a selectivity of 68.6%. Only slightly increase of acetophenone selectivity was observed during the catalytic process. The above results suggest that the active species resulting in the styrene oxide is formed during the catalytic process.
Recycle tests were conducted to assess the recyclability of Au/HNS catalyst, because durability is an important parameter of heterogeneous catalysts. After each cycle, the catalyst was separated by centrifugation, washed with dioxide, and used for the next run. The recycling results are displayed in Fig. 4b. No obvious deterioration of activity and selectivity was observed after even 8 cycles. After recycling, the ICP-AES was performed to determine the leaching of metal contents and the result indicates that 90.5% of the gold was remained on the catalyst. Notably, the hollow nanostructure and small Au nanoparticle sizes could be remained after recycling though a little increase in size was observed (Fig. 3g). These results suggest that Au/HNS is robust catalyst to be stably recycled.
3. Experimental
3.1 Chemicals and materials
All chemicals were used as received without any further purification. Pluronic F127 (Mw = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600, EO106PO70EO106) was purchased from Sigma Aldrich. (3-Mercaptopropyl) trimethoxysilane (MPTMS) was purchased from Alfa Aesar. HAuCl4·4H2O was purchased from Aladdin-reagent Company. Other reagents were purchased from Shanghai Chemical Reagent Company of the Chinese Medicine Group.
600, EO106PO70EO106) was purchased from Sigma Aldrich. (3-Mercaptopropyl) trimethoxysilane (MPTMS) was purchased from Alfa Aesar. HAuCl4·4H2O was purchased from Aladdin-reagent Company. Other reagents were purchased from Shanghai Chemical Reagent Company of the Chinese Medicine Group.
3.2 Synthesis of hollow silica nanospheres
Typically, 0.40 g F127 and 1.40 g K2SO4 were dissolved in 24 mL of deionized water at 13.5 °C under vigorous stirring. Then, the mixture of 0.40 g trimethylbenzene (TMB) and 0.20 g MPTMS were added quickly. After pre-hydrolysis for 3 h, 1.20 g of tetramethoxysilane (TMOS) was added. The molar composition of the mixture was TMOS/F127/MPTMS/TMB/K2SO4/H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0040
0.0040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13
0.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.42
0.42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.02
1.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 169. The resultant mixture was stirred at 13.5 °C for 24 h and aged at 100 °C under static conditions for an additional 24 h. The solid product was recovered by filtration and air-dried at room temperature overnight. The surfactant was extracted by refluxing 1.0 g of as-synthesized material in 200 mL of ethanol containing 1.5 g of HCl (36.5 wt%) for 24 h. After filtration and washed thoroughly with water and ethanol, the sample was dried and denoted as SH-HNS.
169. The resultant mixture was stirred at 13.5 °C for 24 h and aged at 100 °C under static conditions for an additional 24 h. The solid product was recovered by filtration and air-dried at room temperature overnight. The surfactant was extracted by refluxing 1.0 g of as-synthesized material in 200 mL of ethanol containing 1.5 g of HCl (36.5 wt%) for 24 h. After filtration and washed thoroughly with water and ethanol, the sample was dried and denoted as SH-HNS.
The pure silica hollow nanospheres, HNS, were also synthesized as above without the additive of MPTMS, and the surfactant was removed by calcination.
3.3 Loading ultra-small Au nanoparticles onto SH-HNS and HNS
The solid catalysts with supported Au nanoparticles were prepared as follows: 0.40 g of SH-HNS was dispersed into 20 mL aqueous solution containing desired amount of HAuCl4·4H2O (2, 4, 8, 20 or 32 mg Au). After ultrasonic treatment for 5 min, the solution was stirred for 12 h at room temperature. The solid material was then separated by centrifugation, washed sequentially with distilled water for several times and dried at 100 °C overnight. Afterwards, the reduction step was carried out in the glass tube furnace using H2. The sample was heated to 200 °C with a rate of 1.0 °C min−1 and kept at this temperature for 2 h. The obtained solid catalyst was donated as Au/SH-HNS.Au/SH-HNS-air was obtained similarly with above except that the solid material was firstly calcinated at 350 °C for 3 h under air atmosphere to remove –SH groups before reduction in H2.
Au/HNS catalyst was prepared through an impregnation method: 0.40 g of HNS was dispersed into 2 mL aqueous solution containing 16 mg of Au (HAuCl4·4H2O as precursor). After ultrasonic treatment for 5 min, the mixture was stirred for 12 h at room temperature. The solvent was then removed by rotation evaporation at room temperature. Afterwards, the reduction with H2 was carried out under the same conditions as mentioned above, and the obtained solid catalyst was donated as Au/HNS. For comparison, SBA-15 was also used as support, and the obtained sample was donated as Au/SBA-15.
3.4 Characterization
Nitrogen physical adsorption measurement was carried out on micrometritics ASAP2020 volumetric adsorption analyzer. Before the measurements, the samples were degassed at 393 K for 5 h. The BET surface area was evaluated from the data in the relative pressure range P/P0 of 0.05 to 0.25. The total pore volume was estimated from the amount adsorbed at the P/P0 of 0.99. The pore diameter was determined from the adsorption branch by the BJH method. FT-IR spectra were collected using a Nicolet Nexus 470 IR spectrometer. Transmission electron microscopy (TEM) was performed using a FEI Tecnai G2 Spirit at an acceleration voltage of 120 kV.3.5 The styrene oxidation reaction using O2 as oxidant
Typically, the solid catalyst containing 1.5 μmol Au, 0.66 mL styrene, 10 μL n-decane (as internal standard) and 3 mL dioxane were mixed in a 10 mL Schlenk reaction tube. The atmosphere inside the reaction tube was replaced by flushing with oxygen for over 3 times using an O2 balloon. The reaction was carried on at 100 °C with stirring for desired time. The products were analyzed using a gas chromatography equipped with a HP-5 column. The conversion of styrene was calculated with reference to products formed, containing styrene oxide, benzaldehyde, acetophenone. In the recycle test, catalyst was recovered by centrifugation and washed with dioxane and used in the next run.4. Conclusions
In summary, a unique hollow silica nanosphere with particle size of 20 nm and thin shell thickness was utilized as efficient support for loading Au nanoparticles. It was found that uniform ultra-small Au nanoparticles could be prepared using this support with the aid of chemical modification or through simple immersion method. The solid catalyst showed excellent thermal stability, small particle sizes could be remained even at a high temperature of 350 °C. The obtained solid catalysts with Au NPs could efficiently catalyze the styrene oxidation reaction under 1 atm oxygen gas to afford 45.6% styrene conversion and 68.6% styrene oxide selectivity. Moreover, the solid catalyst could be facilely separated and recycled. This study demonstrates that our hollow nanosphere catalyst has a superior ability in stabilizing metal nanoparticles against growth, which is a crucial factor for designing high-performance catalysts.Acknowledgements
This work was financially supported by NSFC Grant (. 21325313, 21232008, 21321002).Notes and references
- Q. Yue, Y. Zhang, C. Wang, X. Q. Wang, Z. K. Sun, X. F. Hou, D. Y. Zhao and Y. H. Deng, J. Mater. Chem. A, 2015, 3, 4586–4594 CAS.
- N. S. Patil, B. S. Uphade, P. Jana, S. K. Bharagava and V. R. Choudhary, J. Catal., 2004, 223, 236–239 CrossRef CAS.
- V. R. Choudhary, D. K. Dumbre, N. S. Patil, B. S. Uphade and S. K. Bhargava, J. Catal., 2013, 300, 217–224 CrossRef.
- Y. M. Liu, H. Tsunoyama, T. Akita and T. Tsukuda, Chem. Commun., 2010, 46, 550–552 RSC.
- L. Wang, B. S. Zhang, W. Zhang, J. Zhang, X. H. Gao, X. J. Meng, D. S. Su and F. S. Xiao, Chem. Commun., 2013, 49, 3449–3451 RSC.
- R. L. Oliveira, P. K. Kiyohara and L. M. Rossi, Green Chem., 2009, 11, 1366–1370 RSC.
- T. Hayashi, K. Tanaka and M. Haruta, J. Catal., 1998, 178, 566–575 CrossRef CAS.
- Y. Yao, X. M. Zhang, J. Peng and Q. H. Yang, Chem. Commun., 2015, 51, 3750–3753 RSC.
- F. X. Zhu, W. Wang and H. X. Li, J. Am. Chem. Soc., 2011, 133, 11632–11640 CrossRef CAS PubMed.
- L. Wang, H. Wang, P. Hapala, L. F. Zhu, L. M. Ren, X. J. Meng, J. P. Lewis and F. S. Xiao, J. Catal., 2011, 281, 30–39 CrossRef CAS.
- G. C. Ma, A. Binder, M. F. Chi, C. Liu, R. C. Jin, D. E. Jiang, J. Fan and S. Dai, Chem. Commun., 2012, 48, 11413–11415 RSC.
- G. Budroni and A. Corma, Angew. Chem., Int. Ed., 2006, 45, 6244 CrossRef CAS.
- B. Laursen, K. T. Hojholt, L. F. Lundegaard, S. B. Simonsen, S. Helveg, F. Schuth, M. Paul, J. D. Grunwaldt, S. Kegnoes, C. H. Christensen and K. Egeblad, Angew. Chem., Int. Ed., 2010, 49, 3504–3507 CrossRef PubMed.
- P. M. Arnal, M. Comotti and F. Schuth, Angew. Chem., Int. Ed., 2006, 45, 8224–8227 CrossRef CAS PubMed.
- X. C. Kang, J. L. Zhang, W. T. Shang, T. B. Wu, P. Zhang, B. X. Han, Z. H. Wu, G. Mo and X. Q. Xing, J. Am. Chem. Soc., 2014, 136, 3768–3771 CrossRef CAS PubMed.
- H. Tsunoyama, N. Ichikuni, H. Sakurai and T. Tsukuda, J. Am. Chem. Soc., 2009, 131, 7086–7093 CrossRef CAS PubMed.
- Y. Yu, C. Y. Cao, Z. Chen, H. Liu, P. Li, Z. F. Dou and W. G. Song, Chem. Commun., 2013, 49, 3116–3118 RSC.
- L. F. Chen, J. C. Hu and R. Richards, J. Am. Chem. Soc., 2009, 131, 914 CrossRef CAS PubMed.
- E. Besson, A. Mehdi, C. Reye and R. J. P. Corriu, J. Mater. Chem., 2009, 19, 4746–4752 RSC.
- S. Wang, Q. F. Zhao, H. M. Wei, J. Q. Wang, M. Y. Cho, H. S. Cho, O. Terasaki and Y. Wan, J. Am. Chem. Soc., 2013, 135, 11849–11860 CrossRef CAS PubMed.
- S. H. Wu, C. T. Tseng, Y. S. Lin, C. H. Lin, Y. Hung and C. Y. Mou, J. Mater. Chem., 2011, 21, 789–794 RSC.
- T. T. Zhang, H. Y. Zhao, S. N. He, K. Liu, H. Y. Liu, Y. D. Yin and C. B. Gao, ACS Nano, 2014, 8, 7297–7304 CrossRef CAS.
- Y. Y. Yin, M. Chen, S. X. Zhou and L. M. Wu, J. Mater. Chem., 2012, 22, 11245–11251 RSC.
- Y. J. Hao, Y. Z. Chong, S. R. Li and H. Q. Yang, J. Phys. Chem. C, 2012, 116, 6512–6519 CAS.
- X. Q. Yan, X. J. Wang, Y. Tang, G. C. Ma, S. H. Zou, R. H. Li, X. G. Peng, S. Dai and J. Fan, Chem. Mater., 2013, 25, 1556–1563 CrossRef CAS.
- J. Liu, F. Fan, Z. Feng, L. Zhang, S. Bai, Q. Yang and C. Li, J. Phys. Chem. C, 2008, 112, 16445–16451 CAS.
Footnote |
| † These authors contributed equally to this research. |
| This journal is © The Royal Society of Chemistry 2015 |