Highly diastereoselective synthesis of imidazolidine-dispirooxindoles via three-component [3 + 2] cycloadditions of isatins, 2-(aminomethyl)pyridine and isatin-based imines†
Hong-Wu Zhao*,
Xiao-Qin Chen,
Zhao Yang,
Ting Tian,
Bo Li,
Wei Meng,
Xiu-Qing Song and
Hai-Liang Pang
College of Life Science and Bio-engineering, Beijing University of Technology, No. 100 Pingleyuan, Chaoyang District, Beijing 100124, P. R. China. E-mail: hwzhao@bjut.edu.cn
First published on 26th November 2015
Abstract
In the presence of Et3N, the [3 + 2] cycloaddition of isatins, 2-(aminomethyl)pyridine and isatin-based imines proceeded readily, and furnished novel imidazolidine-dispirooxindoles in up to 84% yield with up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity. The relative configuration of the imidazolidine-dispirooxindoles was firmly confirmed on the basis of X-ray single crystal structure analysis. The reaction mechanism was assumed to account for the diastereoselective formation of the imidazolidine-dispirooxindoles.
1 diastereoselectivity. The relative configuration of the imidazolidine-dispirooxindoles was firmly confirmed on the basis of X-ray single crystal structure analysis. The reaction mechanism was assumed to account for the diastereoselective formation of the imidazolidine-dispirooxindoles.
Introduction
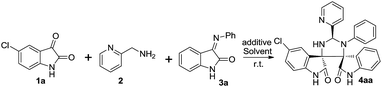
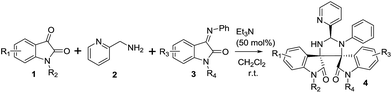
Dispirooxindoles constitute a class of structurally and stereochemically diverse and complex chemical entities, and exhibit a wide range of important biological activities, for example, antidiabetic, anticancer, antitubercular, antibacterial as well as antimicrobial activities as summarized in Fig. 1.1 Since the biological and medicinal importance with dispirooxindoles, organic chemists and medicinal chemists have been involved in developing useful and powerful methodologies for the synthesis of dispirooxindoles with potential biological activities.2 Among the known synthetic methodologies, the [3 + 2] cycloaddition of azomethine ylides with various substituted dipolarophiles served as the main tools for the construction of dispirooxindoles.3 In particular, most of the present pyrrolidine-dispirooxindoles were easily and efficiently produced by means of the [3 + 2] cycloaddition of isatin-derived azomethine ylides with various olefin-based dipolarophiles.4 In spite of the progress made in the synthesis of an array of dispirooxindoles, it is still highly needed to develop more efficient and concise methodologies for the synthesis of dispirooxindoles bearing structural and stereochemical diversity.In the recent years, the diastereoselective construction of structurally unique dispirooxindoles consisting of two spirooxindole motifs has gained attentions from several research groups. For example, in 2012 Yuan and co-workers reported the diastereoselective synthesis of dispiro[imidazolidine-2-thione]bisoxindoles through [3 + 2] cycloaddition of 3-isothiocyanato oxindoles with isatinimines.5 In the same year, Taylor research group carried out the diastereoselective construction of spirocyclic bisoxindoles via a double C–H, Ar–H coupling process.6 In 2015, by following a similar self-cycloaddition strategy, Yang7 and Thennarsu8 research groups individually contributed to the first diastereoselective synthesis of imidazolidine-dispirooxindoles including two spirooxindoles at 2,5-positions of imidazolidine ring by treating azomethine ylides with isatin-based imines as shown in Scheme 1 (eqn (1) & (2)). In our work, we first designed the distereoselective three-component [3 + 2] cycloadditions of isatins, 2-(aminomethyl)pyridine and isatin-based imines for the construction of a series of novel imidazolidine-dispirooxindoles bearing two spirooxindoles at 4,5-positions of imidazolidine ring as outlined in Scheme 1 (eqn (3)). The [3 + 2] cycloadditions underwent smoothly, thus providing the designed unprecedented imidazolidine-dispirooxindoles in desirable chemical yields with high diastereoselectivities.
Results and discussion
Initially, under the different reaction conditions, we examined the three-component [3 + 2] cycloaddition of 1a, 2 and 3a as shown in Table 1. Without any additives, we screened a series of solvents, and found that the use of CH2Cl2 gave 4aa in better chemical yield with comparable diastereoselectivity (Table 1, entries 1–5). Moreover, Lewis acids and protonic acids as additives could cause the different loss in chemical yield and diastereoselectivity (Table 1, entries 6–11). At last, we examined a variety of organic bases as additives as well as catalytic loading of Et3N, and discovered that 50 mol% of Et3N behaved most efficiently, and furnished 4aa in 70% yield with 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dr (Table 1, entries 12–23). Considering the chemical yield and diastereoselectivity of 4aa comprehensively, we determined the optimal reaction conditions as below: 1a
8 dr (Table 1, entries 12–23). Considering the chemical yield and diastereoselectivity of 4aa comprehensively, we determined the optimal reaction conditions as below: 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 1
3a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 50 mol% Et3N, CH2Cl2, room temperature.
1, 50 mol% Et3N, CH2Cl2, room temperature.
| Entry | Solvent | Additive | Time (h) | Yieldb (%) | drc |
|---|---|---|---|---|---|
| a Reaction was conducted with 5-Cl-isatin 1a (0.1 mmol), 2-(aminomethyl)pyridine 2 (0.1 mmol), isatin-based imine 3a (0.1 mmol) in the absence or presence of 10 mol% of additive in the indicated solvents (0.5 mL) at room temperature.b Isolated yield.c Determined by 1H NMR spectroscopy. | |||||
| 1 | THF | — | 24 | 42 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
| 2 | Toluene | — | 24 | 15 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 3 | CH2Cl2 | — | 24 | 53 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 4 | CH3CN | — | 24 | 33 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 5 | EtOH | — | 24 | 19 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 6 | CH2Cl2 | CuI | 40 | Trace | — |
| 7 | CH2Cl2 | Yb(OTf)3 | 40 | 31 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 81 |
| 8 | CH2Cl2 | MgSO4 | 40 | 44 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 9 | CH2Cl2 | PhCO2H | 40 | 55 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 10 | CH2Cl2 | 2,2′-Biphenol | 40 | 61 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
| 11 | CH2Cl2 | Stearic acid | 40 | 58 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| 12 | CH2Cl2 | Quinine | 40 | 52 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 13 | CH2Cl2 | Pyridine | 40 | 64 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 14 | CH2Cl2 | DIPEA | 40 | 51 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 15 | CH2Cl2 | DMAP | 40 | 48 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 16 | CH2Cl2 | DBN | 40 | 47 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 17 | CH2Cl2 | DBU | 40 | 35 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 18 | CH2Cl2 | TBD | 40 | 58 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 19 | CH2Cl2 | Et3N (5 mol%) | 72 | 58 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 20 | CH2Cl2 | Et3N (10 mol%) | 72 | 65 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 21 | CH2Cl2 | Et3N (20 mol%) | 72 | 58 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 22 | CH2Cl2 | Et3N (30 mol%) | 72 | 61 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 23 | CH2Cl2 | Et3N (50 mol%) | 72 | 70 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
Subsequently, we extended the reaction scope as outlined in Table 2 under the optimal reaction conditions by using variable substrates 1 and 3. In most cases, the [3 + 2] cycloadditions gave products 4 in the acceptable chemical yields with excellent diastereoselectivities (entries 1–2, 4, 7, 10–24). As for other cases, products 4 were formed in 63–80% chemical yields with 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33–88
33–88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 diastereoselectivities (entries 3, 5–6 and 8–9). Surprisingly, we found that the [3 + 2] cycloaddition of 1a, benzylamine and 3a did not take place at all (entry 25). Moreover, the use of single crystal X-ray analysis determined the relative configuration of 4de as presented in Fig. 2.9 Based on the relative configuration of 4de, the relative configurations of other imidazolidine-dispirooxindoles 4 were similarly assigned as shown in Table 2.
12 diastereoselectivities (entries 3, 5–6 and 8–9). Surprisingly, we found that the [3 + 2] cycloaddition of 1a, benzylamine and 3a did not take place at all (entry 25). Moreover, the use of single crystal X-ray analysis determined the relative configuration of 4de as presented in Fig. 2.9 Based on the relative configuration of 4de, the relative configurations of other imidazolidine-dispirooxindoles 4 were similarly assigned as shown in Table 2.
| Entry | 1 (R1, R2) | 3 (R3, R4) | Yieldb (%) | drc |
|---|---|---|---|---|
| a Reactions were conducted with isatins 1 (0.1 mmol), 2-(aminomethyl)pyridine 2 (0.1 mmol), isatin-based imines 3 (0.1 mmol), Et3N (0.05 mmol) in CH2Cl2 (0.5 mL) at room temperature for 72 h.b Isolated yield.c Determined by 1H NMR spectroscopy.d Benzylamine was used.e No reaction. | ||||
| 1 | 1a (5-Cl, H) | 3a (H, H) | 70 (4aa) | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 2 | 1b (H, H) | 3a (H, H) | 76 (4ba) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 1b (H, H) | 3b (5-MeO, H) | 63 (4bb) | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 4 | 1a (5-Cl, H) | 3c (5-Cl, H) | 77 (4ac) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 1a (5-Cl, H) | 3b (5-MeO, H) | 70 (4ab) | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 6 | 1c (5-MeO, H) | 3c (5-Cl, H) | 83 (4cc) | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
| 7 | 1c (5-MeO, H) | 3b (5-MeO, H) | 61 (4cb) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 1d (H, Bn) | 3a (H, H) | 80 (4da) | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 9 | 1e (H, Me) | 3a (H, H) | 71 (4ea) | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 10 | 1d (H, Bn) | 3d (H, Me) | 78 (4dd) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 1b (H, H) | 3d (H, Me) | 63 (4bd) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 1d (H, Bn) | 3e (H, Bn) | 81 (4de) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 1f (5-F, H) | 3e (H, Bn) | 61 (4fe) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 1a (5-Cl, H) | 3e (H, Bn) | 83 (4ae) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | 1g (6-Cl, H) | 3e (H, Bn) | 63 (4ge) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | 1h (5-Br, H) | 3e (H, Bn) | 78 (4he) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17 | 1i (6-Br, H) | 3e (H, Bn) | 61 (4ie) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 18 | 1b (H, H) | 3e (H, Bn) | 84 (4be) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 19 | 1j (5-Me, H) | 3e (H, Bn) | 78 (4je) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 20 | 1c (5-MeO, H) | 3e (H, Bn) | 64 (4ce) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 21 | 1b (H, H) | 3f (5-Cl, Bn) | 84 (4bf) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 22 | 1b (H, H) | 3g (5-Br, Bn) | 81 (4bg) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 23 | 1b (H, H) | 3h (5-Me, Bn) | 77 (4bh) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 24 | 1b (H, H) | 3i (5-MeO, Bn) | 75 (4bi) | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 25d | 1a (5-Cl, H) | 3a (H, H) | NRe | — |
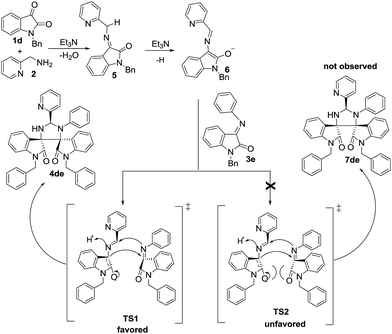
To shed light on the formation and diastereoselectivity of 4de, we proposed the mechanism for the [3 + 2] cycloaddition as illustrated in Scheme 2. Initially, under catalysis of Et3N, isatin 1d condenses easily with 2-(aminomethyl)pyridine 2 to afford imine 5. Subsequently, the deprotonation of imine 5 with Et3N give rise to enolate 6. Finally, the cyclization of the resulted enolate 6 with imine 3e formed diastereoisomer 4de via the transition state TS1. In our case, the formation of diastereoisomer 7de was not observed through the transition state TS2. With the aid of molecular model, it was found that two 2-indolinone rings have a face-to-face overlap in the transition state TS2, and the stronger electronstatic repulsion exists between the two π systems; in comparison, this type of electronstatic repulsion decrease remarkably in the transition state TS1 owing to the parallel-displaced orientation between the two 2-indolinone rings.10 Therefore, the transition state TS1 is more stable than the transition state TS2. Noticeably, the [3 + 2] cycloaddition prefer to adopt the transition state TS1 to lead to formation of thermodynamically more stable diastereoisomer 4de instead of diastereoisomer 7de bearing the severe steric hindrance between the two 2-indolinone moieties. Overall, it was deduced that the preference for the transition state TS1 over the transition state TS2 account for the excellent diastereoselectivity in the [3 + 2] cycloaddition.
Conclusions
In conclusion, by means of Et3N-catalyzed [3 + 2] cycloaddition of isatin-derived azomethine ylides with isatin-based imines, we first accomplished the synthesis of imidazolidine-dispirooxindoles featuring two spirooxindoles at 4,5-positions of the imidazolidine ring. Significantly, our methodology not only enriched the synthetic chemistry of imidazolidine-dispirooxindoles, but also benefited to the discovery of novel dispirooxindoles with potential biological activities. At present, the design and stereoselective synthesis of new type of dispirooxindoles is undergoing in our lab, and will be reported in due course.Experimental section
General methods
Unless noted otherwise, all reagents were commercially available and used without further purification. All solvents were distilled from the appropriate drying agents immediately before use. Reactions were monitored by TLC carried out on 0.25 mm SDS silica gel coated glass plates (60F254) and compounds were detected with UV light. The compound melting point was determined by a melting point instrument. NMR spectra were recorded on 400 MHz instrument and calibrated using tetramethylsilane (TMS) as internal reference. High resolution mass spectra (HRMS) were recorded under electrospray ionization (ESI) conditions.Typical procedure for the diastereoselective synthesis of imidazolidine-dispirooxindoles 4
Triethylamine (0.05 mmol, 6.9 μL, 5.0 mg) was added to a mixture of isatins 1 (0.1 mmol), 2-(aminomethyl)pyridine 2 (0.1 mmol, 9.8 μL, 10.8 mg) and isatin-based imines 3 (0.1 mmol) in anhydrous CH2Cl2 (0.5 mL). The reaction was stirred at room temperature for 72 hours. After completion of the reaction, the crude product was purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford the pure products 4 as white powder (61–84% yield; 67
2) to afford the pure products 4 as white powder (61–84% yield; 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 ≥ 99
33 ≥ 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8; 1H NMR (400 MHz, DMSO): δ 10.90 (s, 1H), 10.51 (s, 1H), 8.58 (s, 1H), 8.46 (d, J = 7.2 Hz, 1H), 7.77 (s, 1H), 7.56 (s, 1H), 7.31–7.25 (m, 2H), 14 (s, 1H), 6.89–6.63 (m, 7H), 6.44 (d, J = 6.0 Hz, 1H), 6.06 (s, 2H), 4.50 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 178.5, 175.9, 160.6, 148.6, 142.7, 142.6, 142.5, 137.6, 130.6, 130.2, 128.6, 127.3, 126.5, 126.0, 125.6, 124.0, 123.6, 122.9, 122.1, 118.0, 115.9, 111.3, 110.6, 79.5, 74.7, 73.5; HRMS (ESI) calculated for C28H21ClN5O2 (M + H+): 494.13783, found 494.13620.
8; 1H NMR (400 MHz, DMSO): δ 10.90 (s, 1H), 10.51 (s, 1H), 8.58 (s, 1H), 8.46 (d, J = 7.2 Hz, 1H), 7.77 (s, 1H), 7.56 (s, 1H), 7.31–7.25 (m, 2H), 14 (s, 1H), 6.89–6.63 (m, 7H), 6.44 (d, J = 6.0 Hz, 1H), 6.06 (s, 2H), 4.50 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 178.5, 175.9, 160.6, 148.6, 142.7, 142.6, 142.5, 137.6, 130.6, 130.2, 128.6, 127.3, 126.5, 126.0, 125.6, 124.0, 123.6, 122.9, 122.1, 118.0, 115.9, 111.3, 110.6, 79.5, 74.7, 73.5; HRMS (ESI) calculated for C28H21ClN5O2 (M + H+): 494.13783, found 494.13620.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.47 (s, 2H), 8.58 (d, J = 4.0 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.79 (t, J = 7.6 Hz, 1H), 7.50 (d, J = 7.6 Hz, 1H), 7.32 (t, J = 5.6 Hz, 1H), 7.20 (t, J = 7.6 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 6.97–6.88 (m, 2H), 6.82–6.61 (m, 6H), 6.43 (t, J = 7.2 Hz, 1H), 6.04 (d, J = 6.0 Hz, 2H), 4.14 (d, J = 5.2 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 178.7, 175.8, 160.6, 148.8, 144.7, 142.7, 142.6, 137.7, 130.7, 128.5, 127.3, 125.9, 124.0, 123.4, 123.3, 123.2, 122.1, 122.0, 117.9, 115.9, 110.5, 109.9, 79.5, 74.9, 73.3; HRMS (ESI) calculated for C28H22N5O2 (M + H+): 460.17680, found 460.17548.
1; 1H NMR (400 MHz, DMSO): δ 10.47 (s, 2H), 8.58 (d, J = 4.0 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.79 (t, J = 7.6 Hz, 1H), 7.50 (d, J = 7.6 Hz, 1H), 7.32 (t, J = 5.6 Hz, 1H), 7.20 (t, J = 7.6 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 6.97–6.88 (m, 2H), 6.82–6.61 (m, 6H), 6.43 (t, J = 7.2 Hz, 1H), 6.04 (d, J = 6.0 Hz, 2H), 4.14 (d, J = 5.2 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 178.7, 175.8, 160.6, 148.8, 144.7, 142.7, 142.6, 137.7, 130.7, 128.5, 127.3, 125.9, 124.0, 123.4, 123.3, 123.2, 122.1, 122.0, 117.9, 115.9, 110.5, 109.9, 79.5, 74.9, 73.3; HRMS (ESI) calculated for C28H22N5O2 (M + H+): 460.17680, found 460.17548.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15; 1H NMR (400 MHz, DMSO): δ 10.63 (s, 1H), 10.48 (s, 1H), 8.58 (d, J = 4.0 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.31 (t, J = 5.6 Hz, 1H), 7.19 (d, J = 8.0 Hz, 1H), 6.95 (t, J = 7.6 Hz, 1H), 6.82 (t, J = 7.6 Hz, 2H), 6.72–6.62 (m, 4H), 6.54 (s, 1H), 6.45 (t, J = 7.2 Hz, 2H), 6.06 (d, J = 6.8 Hz, 2H), 4.11 (d, J = 9.2 Hz, 1H), 3.52 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.5, 175.7, 160.6, 154.8, 148.8, 143.5, 142.7, 137.7, 136.0, 130.7, 128.6, 125.9, 124.6, 124.0, 123.4, 123.3, 122.1, 117.9, 115.9, 114.8, 113.9, 110.7, 109.9, 79.6, 75.0, 73.4, 55.7; HRMS (ESI) calculated for C29H24N5O3 (M + H+): 490.18737, found 490.18607.
15; 1H NMR (400 MHz, DMSO): δ 10.63 (s, 1H), 10.48 (s, 1H), 8.58 (d, J = 4.0 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.31 (t, J = 5.6 Hz, 1H), 7.19 (d, J = 8.0 Hz, 1H), 6.95 (t, J = 7.6 Hz, 1H), 6.82 (t, J = 7.6 Hz, 2H), 6.72–6.62 (m, 4H), 6.54 (s, 1H), 6.45 (t, J = 7.2 Hz, 2H), 6.06 (d, J = 6.8 Hz, 2H), 4.11 (d, J = 9.2 Hz, 1H), 3.52 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.5, 175.7, 160.6, 154.8, 148.8, 143.5, 142.7, 137.7, 136.0, 130.7, 128.6, 125.9, 124.6, 124.0, 123.4, 123.3, 122.1, 117.9, 115.9, 114.8, 113.9, 110.7, 109.9, 79.6, 75.0, 73.4, 55.7; HRMS (ESI) calculated for C29H24N5O3 (M + H+): 490.18737, found 490.18607.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 11.04 (s, 1H), 10.69 (s, 1H), 8.56 (d, J = 4.4 Hz, 1H), 8.42 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.53 (d, J = 2.0 Hz, 1H), 7.33–7.20 (m, 3H), 6.87–6.94 (m, 3H), 6.76 (d, J = 8.4 Hz, 1H), 6.68–6.63 (m, 2H), 6.49 (t, J = 7.2 Hz, 1H), 6.06 (d, J = 6.8 Hz, 2H), 4.61 (d, J = 8.8 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 178.1, 175.9, 160.3, 148.6, 142.3, 141.7, 137.6, 130.8, 130.1, 128.8, 126.9, 126.5, 126.2, 126.1, 125.2, 125.1, 124.1, 126.6, 118.5, 115.9, 112.1, 111.4, 79.5, 75.0, 73.4; HRMS (ESI) calculated for C28H20Cl2N5O2 (M + H+): 528.09886, found 528.09767.
1; 1H NMR (400 MHz, DMSO): δ 11.04 (s, 1H), 10.69 (s, 1H), 8.56 (d, J = 4.4 Hz, 1H), 8.42 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.53 (d, J = 2.0 Hz, 1H), 7.33–7.20 (m, 3H), 6.87–6.94 (m, 3H), 6.76 (d, J = 8.4 Hz, 1H), 6.68–6.63 (m, 2H), 6.49 (t, J = 7.2 Hz, 1H), 6.06 (d, J = 6.8 Hz, 2H), 4.61 (d, J = 8.8 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 178.1, 175.9, 160.3, 148.6, 142.3, 141.7, 137.6, 130.8, 130.1, 128.8, 126.9, 126.5, 126.2, 126.1, 125.2, 125.1, 124.1, 126.6, 118.5, 115.9, 112.1, 111.4, 79.5, 75.0, 73.4; HRMS (ESI) calculated for C28H20Cl2N5O2 (M + H+): 528.09886, found 528.09767.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12; 1H NMR (400 MHz, DMSO): δ 10.75 (s, 1H), 10.58 (s, 1H), 8.56 (d, J = 4.4 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.77 (t, J = 8.0 Hz, 1H), 7.55 (d, J = 2.0 Hz, 1H), 7.33–7.25 (m, 2H), 6.82 (t, J = 7.6 Hz, 2H), 6.74–6.71 (m, 1H), 6.66–6.63 (m, 3H), 6.50 (d, J = 2.4 Hz, 1H), 6.45 (t, J = 7.2 Hz, 1H), 6.06 (d, J = 7.2 Hz, 2H), 4.48 (d, J = 8.8 Hz, 1H), 3.52 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.2, 175.9, 160.5, 154.8, 148.6, 142.6, 142.4, 137.6, 135.8, 130.6, 128.6, 126.5, 126.0, 125.5, 124.4, 124.0, 123.6, 118.0, 115.9, 114.8, 114.1, 111.3, 110.8, 79.5, 74.8, 73.5, 55.7; HRMS (ESI) calculated for C29H23ClN5O3 (M + H+): 524.14839, found 524.14740.
12; 1H NMR (400 MHz, DMSO): δ 10.75 (s, 1H), 10.58 (s, 1H), 8.56 (d, J = 4.4 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.77 (t, J = 8.0 Hz, 1H), 7.55 (d, J = 2.0 Hz, 1H), 7.33–7.25 (m, 2H), 6.82 (t, J = 7.6 Hz, 2H), 6.74–6.71 (m, 1H), 6.66–6.63 (m, 3H), 6.50 (d, J = 2.4 Hz, 1H), 6.45 (t, J = 7.2 Hz, 1H), 6.06 (d, J = 7.2 Hz, 2H), 4.48 (d, J = 8.8 Hz, 1H), 3.52 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.2, 175.9, 160.5, 154.8, 148.6, 142.6, 142.4, 137.6, 135.8, 130.6, 128.6, 126.5, 126.0, 125.5, 124.4, 124.0, 123.6, 118.0, 115.9, 114.8, 114.1, 111.3, 110.8, 79.5, 74.8, 73.5, 55.7; HRMS (ESI) calculated for C29H23ClN5O3 (M + H+): 524.14839, found 524.14740.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33; 1H NMR (400 MHz, DMSO): δ 10.96 (s, 1H), 10.41 (s, 1H), 8.58 (d, J = 4.4 Hz, 1H), 8.41 (d, J = 7.6 Hz, 1H), 7.79 (t, J = 7.2 Hz, 1H), 7.34–7.28 (m, 1H), 7.23–7.16 (m, 2H), 6.87–6.73 (m, 5H), 6.68–6.63 (m, 1H), 6.57 (d, J = 8.4 Hz, 1H), 6.48 (t, J = 7.6 Hz, 1H), 6.06 (s 2H), 4.30 (d, J = 9.2 Hz, 1H), 3.70 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.3, 175.8, 160.3, 155.1, 148.8, 142.5, 141.8, 137.7, 136.4, 129.9, 128.8, 127.0, 126.0, 125.4, 125.2, 124.2, 124.1, 123.4, 118.3, 115.9, 115.4, 113.3, 112.0, 110.3, 79.7, 75.4, 73.4, 55.9; HRMS (ESI) calculated for C29H23ClN5O3 (M + H+): 524.14839, found 524.14746.
33; 1H NMR (400 MHz, DMSO): δ 10.96 (s, 1H), 10.41 (s, 1H), 8.58 (d, J = 4.4 Hz, 1H), 8.41 (d, J = 7.6 Hz, 1H), 7.79 (t, J = 7.2 Hz, 1H), 7.34–7.28 (m, 1H), 7.23–7.16 (m, 2H), 6.87–6.73 (m, 5H), 6.68–6.63 (m, 1H), 6.57 (d, J = 8.4 Hz, 1H), 6.48 (t, J = 7.6 Hz, 1H), 6.06 (s 2H), 4.30 (d, J = 9.2 Hz, 1H), 3.70 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.3, 175.8, 160.3, 155.1, 148.8, 142.5, 141.8, 137.7, 136.4, 129.9, 128.8, 127.0, 126.0, 125.4, 125.2, 124.2, 124.1, 123.4, 118.3, 115.9, 115.4, 113.3, 112.0, 110.3, 79.7, 75.4, 73.4, 55.9; HRMS (ESI) calculated for C29H23ClN5O3 (M + H+): 524.14839, found 524.14746.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.66 (s, 1H), 10.30 (s, 1H), 8.58 (d, J = 4.0 Hz, 1H), 8.44 (d, J = 7.6 Hz, 1H), 7.78 (t, J = 7.2 Hz, 1H), 7.32 (t, J = 2.0 Hz, 1H), 7.18 (s, 1H), 6.84–6.63 (m, 6H), 6.54 (s, 2H), 6.44 (t, J = 7.2 Hz, 1H), 6.05 (d, J = 7.2 Hz, 2H), 4.14 (d, J = 9.2 Hz, 1H), 3.69 (s, 3H), 3.51 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.5, 175.7, 160.5, 155.0, 154.8, 148.8, 142.7, 137.7, 136.5, 135.9, 128.6, 124.6, 124.5, 124.0, 123.3, 117.9, 115.9, 115.2, 114.9, 113.9, 113.3, 110.7, 110.2, 79.7, 75.3, 73.5, 55.9, 55.7; HRMS (ESI) calculated for C30H26N5O4 (M + H+): 520.19793, found 520.19666.
1; 1H NMR (400 MHz, DMSO): δ 10.66 (s, 1H), 10.30 (s, 1H), 8.58 (d, J = 4.0 Hz, 1H), 8.44 (d, J = 7.6 Hz, 1H), 7.78 (t, J = 7.2 Hz, 1H), 7.32 (t, J = 2.0 Hz, 1H), 7.18 (s, 1H), 6.84–6.63 (m, 6H), 6.54 (s, 2H), 6.44 (t, J = 7.2 Hz, 1H), 6.05 (d, J = 7.2 Hz, 2H), 4.14 (d, J = 9.2 Hz, 1H), 3.69 (s, 3H), 3.51 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.5, 175.7, 160.5, 155.0, 154.8, 148.8, 142.7, 137.7, 136.5, 135.9, 128.6, 124.6, 124.5, 124.0, 123.3, 117.9, 115.9, 115.2, 114.9, 113.9, 113.3, 110.7, 110.2, 79.7, 75.3, 73.5, 55.9, 55.7; HRMS (ESI) calculated for C30H26N5O4 (M + H+): 520.19793, found 520.19666.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25; 1H NMR (400 MHz, DMSO): δ 10.77 (s, 1H), 8.58 (d, J = 4.8 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.34 (t, J = 4.8 Hz, 1H), 7.20–7.16 (m, 4H), 7.02 (t, J = 7.6 Hz, 1H), 6.85–6.71 (m, 9H), 6.53 (d, J = 8.0 Hz, 1H), 6.45 (t, J = 7.2 Hz, 1H), 6.07 (d, J = 7.2 Hz, 2H), 4.88 (d, J = 16.0 Hz, 1H), 4.52 (d, J = 16.0 Hz, 1H), 4.35 (d, J = 9.2 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 178.7, 174.2, 160.6, 148.7, 143.9, 142.9, 142.6, 137.3, 135.7, 130.8, 130.0, 128.9, 127.6, 127.5, 127.3, 125.9, 124.1, 123.4, 123.1, 123.0, 122.9, 122.2, 118.1, 116.0, 110.7, 109.7, 79.5, 74.7, 73.6, 42.7; HRMS (ESI) calculated for C35H28N5O2 (M + H+): 550.22375, found 550.22229.
25; 1H NMR (400 MHz, DMSO): δ 10.77 (s, 1H), 8.58 (d, J = 4.8 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.34 (t, J = 4.8 Hz, 1H), 7.20–7.16 (m, 4H), 7.02 (t, J = 7.6 Hz, 1H), 6.85–6.71 (m, 9H), 6.53 (d, J = 8.0 Hz, 1H), 6.45 (t, J = 7.2 Hz, 1H), 6.07 (d, J = 7.2 Hz, 2H), 4.88 (d, J = 16.0 Hz, 1H), 4.52 (d, J = 16.0 Hz, 1H), 4.35 (d, J = 9.2 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 178.7, 174.2, 160.6, 148.7, 143.9, 142.9, 142.6, 137.3, 135.7, 130.8, 130.0, 128.9, 127.6, 127.5, 127.3, 125.9, 124.1, 123.4, 123.1, 123.0, 122.9, 122.2, 118.1, 116.0, 110.7, 109.7, 79.5, 74.7, 73.6, 42.7; HRMS (ESI) calculated for C35H28N5O2 (M + H+): 550.22375, found 550.22229.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24; 1H NMR (400 MHz, DMSO): δ 10.76 (s, 1H), 8.58 (d, J = 4.0 Hz, 1H), 8.47 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.55 (d, J = 7.6 Hz, 1H), 7.33–7.28 (m, 2H), 7.10 (t, J = 7.6 Hz, 1H), 7.03 (t, J = 7.6 Hz, 1H), 6.82–6.67 (m, 7H), 6.43 (t, J = 7.2 Hz, 1H), 6.06 (d, J = 7.2 Hz, 2H), 4.27 (d, J = 9.2 Hz, 1H), 2.85 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.4, 174.2, 160.6, 148.8, 144.7, 142.7, 142.6, 137.7, 130.9, 130.1, 128.6, 126.8, 125.5, 124.1, 123.4, 122.9, 122.7, 122.6, 121.7, 118.0, 115.9, 110.5, 108.9, 79.7, 74.9, 73.5, 25.8; HRMS (ESI) calculated for C29H24N5O2 (M + H+): 474.19245, found 474.19119.
24; 1H NMR (400 MHz, DMSO): δ 10.76 (s, 1H), 8.58 (d, J = 4.0 Hz, 1H), 8.47 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.55 (d, J = 7.6 Hz, 1H), 7.33–7.28 (m, 2H), 7.10 (t, J = 7.6 Hz, 1H), 7.03 (t, J = 7.6 Hz, 1H), 6.82–6.67 (m, 7H), 6.43 (t, J = 7.2 Hz, 1H), 6.06 (d, J = 7.2 Hz, 2H), 4.27 (d, J = 9.2 Hz, 1H), 2.85 (s, 3H); 13C NMR (100 MHz, DMSO): δ 178.4, 174.2, 160.6, 148.8, 144.7, 142.7, 142.6, 137.7, 130.9, 130.1, 128.6, 126.8, 125.5, 124.1, 123.4, 122.9, 122.7, 122.6, 121.7, 118.0, 115.9, 110.5, 108.9, 79.7, 74.9, 73.5, 25.8; HRMS (ESI) calculated for C29H24N5O2 (M + H+): 474.19245, found 474.19119.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 8.59 (d, J = 4.0 Hz, 1H), 8.55 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 7.2 Hz, 1H), 7.53 (d, J = 7.6 Hz, 1H), 7.35–7.26 (m, 2H), 7.19–7.16 (m, 4H), 7.02–6.95 (m, 2H), 6.88–6.76 (m, 7H), 6.53 (d, J = 7.6 Hz, 1H), 6.45 (t, J = 7.6 Hz, 1H), 6.04 (d, J = 6.8 Hz, 2H), 4.86 (d, J = 15.6 Hz, 1H), 4.52 (d, J = 15.6 Hz, 1H), 4.47 (d, J = 8.8 Hz, 1H), 3.08 (s, 3H); 13C NMR (100 MHz, DMSO): δ 176.9, 174.1, 160.5, 148.7, 144.1, 143.9, 142.5, 137.7, 135.7, 130.9, 130.1, 128.9, 128.6, 127.6, 127.3, 126.9, 125.5, 124.1, 123.5, 122.8, 122.7, 122.6, 122.4, 118.2, 116.2, 109.7, 109.6, 79.6, 74.9, 73.5, 42.7, 26.5; HRMS (ESI) calculated for C36H30N5O2 (M + H+): 564.23940, found 564.23779.
1; 1H NMR (400 MHz, DMSO): δ 8.59 (d, J = 4.0 Hz, 1H), 8.55 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 7.2 Hz, 1H), 7.53 (d, J = 7.6 Hz, 1H), 7.35–7.26 (m, 2H), 7.19–7.16 (m, 4H), 7.02–6.95 (m, 2H), 6.88–6.76 (m, 7H), 6.53 (d, J = 7.6 Hz, 1H), 6.45 (t, J = 7.6 Hz, 1H), 6.04 (d, J = 6.8 Hz, 2H), 4.86 (d, J = 15.6 Hz, 1H), 4.52 (d, J = 15.6 Hz, 1H), 4.47 (d, J = 8.8 Hz, 1H), 3.08 (s, 3H); 13C NMR (100 MHz, DMSO): δ 176.9, 174.1, 160.5, 148.7, 144.1, 143.9, 142.5, 137.7, 135.7, 130.9, 130.1, 128.9, 128.6, 127.6, 127.3, 126.9, 125.5, 124.1, 123.5, 122.8, 122.7, 122.6, 122.4, 118.2, 116.2, 109.7, 109.6, 79.6, 74.9, 73.5, 42.7, 26.5; HRMS (ESI) calculated for C36H30N5O2 (M + H+): 564.23940, found 564.23779.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.44 (s, 1H), 8.59 (d, J = 4.4 Hz, 1H), 8.50 (d, J = 8.0 Hz, 1H), 7.79 (t, J = 7.6 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.33 (t, J = 5.6 Hz, 1H), 7.24–7.16 (m, 2H), 6.92 (d, J = 7.2 Hz, 3H), 6.87–6.77 (m, 3H), 6.72 (d, J = 9.2 Hz, 1H), 6.59 (d, J = 7.6 Hz, 1H), 6.43 (t, J = 8.0 Hz, 1H), 6.00 (d, J = 7.2 Hz, 2H), 4.24 (d, J = 9.2 Hz, 1H), 3.07 (s, 3H); 13C NMR (100 MHz, DMSO): δ 176.9, 175.7, 160.6, 148.8, 144.0, 143.4, 142.6, 137.7, 130.8, 130.1, 128.6, 126.8, 125.4, 124.1, 123.4, 123.1, 122.6, 122.5, 121.8, 118.0, 117.7, 116.0, 110.0, 109.4, 79.6, 75.1, 73.3, 26.4; HRMS (ESI) calculated for C29H24N5O2 (M + H+): 474.19245, found 474.19101.
1; 1H NMR (400 MHz, DMSO): δ 10.44 (s, 1H), 8.59 (d, J = 4.4 Hz, 1H), 8.50 (d, J = 8.0 Hz, 1H), 7.79 (t, J = 7.6 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.33 (t, J = 5.6 Hz, 1H), 7.24–7.16 (m, 2H), 6.92 (d, J = 7.2 Hz, 3H), 6.87–6.77 (m, 3H), 6.72 (d, J = 9.2 Hz, 1H), 6.59 (d, J = 7.6 Hz, 1H), 6.43 (t, J = 8.0 Hz, 1H), 6.00 (d, J = 7.2 Hz, 2H), 4.24 (d, J = 9.2 Hz, 1H), 3.07 (s, 3H); 13C NMR (100 MHz, DMSO): δ 176.9, 175.7, 160.6, 148.8, 144.0, 143.4, 142.6, 137.7, 130.8, 130.1, 128.6, 126.8, 125.4, 124.1, 123.4, 123.1, 122.6, 122.5, 121.8, 118.0, 117.7, 116.0, 110.0, 109.4, 79.6, 75.1, 73.3, 26.4; HRMS (ESI) calculated for C29H24N5O2 (M + H+): 474.19245, found 474.19101.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 8.60 (d, J = 4.0 Hz, 1H), 8.53 (d, J = 8.0 Hz, 1H), 7.82 (t, J = 7.2 Hz, 1H), 7.56 (d, J = 7.6 Hz, 1H), 7.35–7.32 (m, 1H), 7.25–7.10 (m, 10H), 6.94–6.91 (m, 2H), 6.84–6.72 (m, 7H), 6.57 (d, J = 8.0 Hz, 1H), 6.44 (t, J = 8.8 Hz, 1H), 6.05 (d, J = 7.2 Hz, 2H), 4.90 (s, 2H), 4.86 (d, J = 16.0 Hz, 1H), 4.56 (d, J = 8.4 Hz, 1H), 4.54 (d, J = 16.0 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 177.1, 174.5, 160.6, 148.7, 144.0, 143.6, 142.4, 137.8, 136.2, 135.7, 130.8, 130.1, 129.0, 128.5, 127.9, 127.8, 127.6, 127.3, 126.0, 124.1, 123.5, 123.0, 122.9, 122.8, 122.7, 118.3, 116.2, 110.4, 109.8, 79.6, 74.9, 73.5, 43.6, 42.7; HRMS (ESI) calculated for C42H34N5O2 (M + H+): 640.27070, found 640.26917.
1; 1H NMR (400 MHz, DMSO): δ 8.60 (d, J = 4.0 Hz, 1H), 8.53 (d, J = 8.0 Hz, 1H), 7.82 (t, J = 7.2 Hz, 1H), 7.56 (d, J = 7.6 Hz, 1H), 7.35–7.32 (m, 1H), 7.25–7.10 (m, 10H), 6.94–6.91 (m, 2H), 6.84–6.72 (m, 7H), 6.57 (d, J = 8.0 Hz, 1H), 6.44 (t, J = 8.8 Hz, 1H), 6.05 (d, J = 7.2 Hz, 2H), 4.90 (s, 2H), 4.86 (d, J = 16.0 Hz, 1H), 4.56 (d, J = 8.4 Hz, 1H), 4.54 (d, J = 16.0 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 177.1, 174.5, 160.6, 148.7, 144.0, 143.6, 142.4, 137.8, 136.2, 135.7, 130.8, 130.1, 129.0, 128.5, 127.9, 127.8, 127.6, 127.3, 126.0, 124.1, 123.5, 123.0, 122.9, 122.8, 122.7, 118.3, 116.2, 110.4, 109.8, 79.6, 74.9, 73.5, 43.6, 42.7; HRMS (ESI) calculated for C42H34N5O2 (M + H+): 640.27070, found 640.26917.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.43 (s, 1H), 8.58 (d, J = 4.4 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.2 Hz, 1H), 7.34–7.25 (m, 5H), 7.14–7.04 (m, 4H), 6.98 (d, J = 7.6 Hz, 1H), 6.83 (t, J = 7.6 Hz, 1H), 6.77–6.72 (m, 4H), 6.66 (m, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.02 (d, J = 7.2 Hz, 2H), 5.05 (d, J = 15.6 Hz, 1H), 4.84 (d, J = 15.6 Hz, 1H), 4.62 (d, J = 8.8 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.4, 160.6, 157.0, 148.6, 143.6, 142.4, 139.7, 137.7, 136.1, 130.2, 129.0, 128.5, 127.9, 127.4, 127.2, 124.1, 123.6, 122.9, 122.6, 118.3, 116.3, 110.3, 79.3, 75.2, 73.4, 43.6; HRMS (ESI) calculated for C35H27FN5O2 (M + H+): 568.21433, found 568.21271.
1; 1H NMR (400 MHz, DMSO): δ 10.43 (s, 1H), 8.58 (d, J = 4.4 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.2 Hz, 1H), 7.34–7.25 (m, 5H), 7.14–7.04 (m, 4H), 6.98 (d, J = 7.6 Hz, 1H), 6.83 (t, J = 7.6 Hz, 1H), 6.77–6.72 (m, 4H), 6.66 (m, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.02 (d, J = 7.2 Hz, 2H), 5.05 (d, J = 15.6 Hz, 1H), 4.84 (d, J = 15.6 Hz, 1H), 4.62 (d, J = 8.8 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.4, 160.6, 157.0, 148.6, 143.6, 142.4, 139.7, 137.7, 136.1, 130.2, 129.0, 128.5, 127.9, 127.4, 127.2, 124.1, 123.6, 122.9, 122.6, 118.3, 116.3, 110.3, 79.3, 75.2, 73.4, 43.6; HRMS (ESI) calculated for C35H27FN5O2 (M + H+): 568.21433, found 568.21271.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.54 (s, 1H), 8.58 (d, J = 4.0 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.54 (s, 1H), 7.34–7.26 (m, 5H), 7.14 (t, J = 7.6 Hz, 1H), 7.04 (s, 2H), 6.96 (d, J = 7.6 Hz, 1H), 6.82 (d, J = 7.6 Hz, 1H), 6.77–6.66 (m, 5H), 6.43 (t, J = 7.2 Hz, 1H), 6.02 (d, J = 6.0 Hz, 2H), 5.08 (d, J = 15.6 Hz, 1H), 4.79 (d, J = 15.6 Hz, 1H), 4.73 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.3, 160.6, 148.6, 143.6, 142.6, 142.4, 137.6, 136.1, 130.7, 130.2, 129.1, 128.5, 127.9, 127.4, 127.2, 126.8, 126.4, 125.6, 124.0, 123.7, 122.9, 122.6, 118.3, 116.2, 111.4, 110.3, 79.7, 74.9, 73.5, 43.6; HRMS (ESI) calculated for C35H27ClN5O2 (M + H+): 584.18478, found 584.18353.
1; 1H NMR (400 MHz, DMSO): δ 10.54 (s, 1H), 8.58 (d, J = 4.0 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.54 (s, 1H), 7.34–7.26 (m, 5H), 7.14 (t, J = 7.6 Hz, 1H), 7.04 (s, 2H), 6.96 (d, J = 7.6 Hz, 1H), 6.82 (d, J = 7.6 Hz, 1H), 6.77–6.66 (m, 5H), 6.43 (t, J = 7.2 Hz, 1H), 6.02 (d, J = 6.0 Hz, 2H), 5.08 (d, J = 15.6 Hz, 1H), 4.79 (d, J = 15.6 Hz, 1H), 4.73 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.3, 160.6, 148.6, 143.6, 142.6, 142.4, 137.6, 136.1, 130.7, 130.2, 129.1, 128.5, 127.9, 127.4, 127.2, 126.8, 126.4, 125.6, 124.0, 123.7, 122.9, 122.6, 118.3, 116.2, 111.4, 110.3, 79.7, 74.9, 73.5, 43.6; HRMS (ESI) calculated for C35H27ClN5O2 (M + H+): 584.18478, found 584.18353.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.55 (s, 1H), 8.58 (d, J = 4.4 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.34–7.27 (m, 4H), 7.15 (t, J = 7.6 Hz, 1H), 7.06 (d, J = 2.0 Hz, 2H), 6.94 (d, J = 7.2 Hz, 1H), 6.86–6.71 (m, 6H), 6.67 (s, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.02 (d, J = 7.2 Hz, 2H), 4.98 (d, J = 15.6 Hz, 1H), 4.85 (d, J = 15.6 Hz, 1H), 4.53 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.4, 160.7, 148.6, 145.0, 143.5, 142.4, 137.7, 136.2, 135.2, 130.2, 128.9, 128.5, 127.9, 127.8, 127.7, 127.1, 124.1, 123.6, 122.8, 122.6, 122.4, 122.0, 118.3, 116.1, 110.3, 110.0, 79.5, 74.6, 73.3, 43.6; HRMS (ESI) calculated for C35H27ClN5O2 (M + H+): 584.18478, found 584.18384.
1; 1H NMR (400 MHz, DMSO): δ 10.55 (s, 1H), 8.58 (d, J = 4.4 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.34–7.27 (m, 4H), 7.15 (t, J = 7.6 Hz, 1H), 7.06 (d, J = 2.0 Hz, 2H), 6.94 (d, J = 7.2 Hz, 1H), 6.86–6.71 (m, 6H), 6.67 (s, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.02 (d, J = 7.2 Hz, 2H), 4.98 (d, J = 15.6 Hz, 1H), 4.85 (d, J = 15.6 Hz, 1H), 4.53 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.4, 160.7, 148.6, 145.0, 143.5, 142.4, 137.7, 136.2, 135.2, 130.2, 128.9, 128.5, 127.9, 127.8, 127.7, 127.1, 124.1, 123.6, 122.8, 122.6, 122.4, 122.0, 118.3, 116.1, 110.3, 110.0, 79.5, 74.6, 73.3, 43.6; HRMS (ESI) calculated for C35H27ClN5O2 (M + H+): 584.18478, found 584.18384.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.51 (s, 1H), 8.57 (d, J = 4.4 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.2 Hz, 1H), 7.66 (s, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.34–7.27 (m, 4H), 7.13 (t, J = 7.6 Hz, 1H), 7.05 (s, 2H), 6.95 (d, J = 7.6 Hz, 1H), 6.84–6.70 (m, 5H), 6.62 (d, J = 8.0 Hz, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.01 (d, J = 6.0 Hz, 2H), 5.07 (d, J = 15.6 Hz, 1H), 4.97 (d, J = 15.6 Hz, 1H), 4.73 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.2, 160.6, 148.6, 143.5, 143.0, 142.4, 137.6, 136.1, 133.5, 130.2, 129.5, 129.1, 128.5, 127.9, 127.4, 127.2, 126.0, 124.0, 123.7, 122.9, 122.6, 118.3, 116.1, 114.1, 112.0, 110.3, 79.6, 74.9, 73.5, 43.6; HRMS (ESI) calculated for C35H27BrN5O2 (M + H+): 628.13426, found 628.13330.
1; 1H NMR (400 MHz, DMSO): δ 10.51 (s, 1H), 8.57 (d, J = 4.4 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.2 Hz, 1H), 7.66 (s, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.34–7.27 (m, 4H), 7.13 (t, J = 7.6 Hz, 1H), 7.05 (s, 2H), 6.95 (d, J = 7.6 Hz, 1H), 6.84–6.70 (m, 5H), 6.62 (d, J = 8.0 Hz, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.01 (d, J = 6.0 Hz, 2H), 5.07 (d, J = 15.6 Hz, 1H), 4.97 (d, J = 15.6 Hz, 1H), 4.73 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.2, 160.6, 148.6, 143.5, 143.0, 142.4, 137.6, 136.1, 133.5, 130.2, 129.5, 129.1, 128.5, 127.9, 127.4, 127.2, 126.0, 124.0, 123.7, 122.9, 122.6, 118.3, 116.1, 114.1, 112.0, 110.3, 79.6, 74.9, 73.5, 43.6; HRMS (ESI) calculated for C35H27BrN5O2 (M + H+): 628.13426, found 628.13330.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.53 (s, 1H), 8.57 (d, J = 4.8 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.2 Hz, 1H), 7.79–7.15 (m, 5H), 7.06 (t, J = 7.6 Hz, 1H), 7.05 (d, J = 3.6 Hz, 2H), 7.00 (d, J = 2.0 Hz, 1H), 6.98 (d, J = 1.6 Hz, 1H), 6.95–6.70 (m, 6H), 6.43 (t, J = 7.2 Hz, 1H), 6.01 (d, J = 6.8 Hz, 2H), 5.10 (d, J = 15.6 Hz, 1H), 4.84 (d, J = 15.6 Hz, 1H), 4.53 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.3, 160.7, 148.6, 145.1, 143.5, 142.4, 137.7, 136.2, 130.2, 128.9, 128.5, 128.1, 127.9, 127.8, 127.7, 127.1, 124.9, 124.1, 123.7, 123.5, 122.9, 122.8, 122.6, 118.3, 116.1, 112.8, 110.3, 79.5, 74.7, 73.2, 43.6; HRMS (ESI) calculated for C35H27BrN5O2 (M + H+): 628.13426, found 628.13385.
1; 1H NMR (400 MHz, DMSO): δ 10.53 (s, 1H), 8.57 (d, J = 4.8 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.2 Hz, 1H), 7.79–7.15 (m, 5H), 7.06 (t, J = 7.6 Hz, 1H), 7.05 (d, J = 3.6 Hz, 2H), 7.00 (d, J = 2.0 Hz, 1H), 6.98 (d, J = 1.6 Hz, 1H), 6.95–6.70 (m, 6H), 6.43 (t, J = 7.2 Hz, 1H), 6.01 (d, J = 6.8 Hz, 2H), 5.10 (d, J = 15.6 Hz, 1H), 4.84 (d, J = 15.6 Hz, 1H), 4.53 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.9, 176.3, 160.7, 148.6, 145.1, 143.5, 142.4, 137.7, 136.2, 130.2, 128.9, 128.5, 128.1, 127.9, 127.8, 127.7, 127.1, 124.9, 124.1, 123.7, 123.5, 122.9, 122.8, 122.6, 118.3, 116.1, 112.8, 110.3, 79.5, 74.7, 73.2, 43.6; HRMS (ESI) calculated for C35H27BrN5O2 (M + H+): 628.13426, found 628.13385.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.45 (s, 1H), 8.59 (d, J = 4.0 Hz, 1H), 8.50 (d, J = 8.0 Hz, 1H), 7.81 (t, J = 7.2 Hz, 1H), 7.46 (d, J = 7.2 Hz, 1H), 7.33 (t, J = 5.6 Hz, 1H), 7.25–7.21 (m, 4H), 7.14–7.07 (m, 3H), 6.98 (d, J = 7.6 Hz, 1H), 6.88–6.71 (m, 6H), 6.65 (d, J = 7.6 Hz, 1H), 6.43 (t, J = 6.8 Hz, 1H), 6.10 (d, J = 6.8 Hz, 2H), 4.93 (d, J = 15.6 Hz, 1H), 4.86 (d, J = 15.6 Hz, 1H), 4.35 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 177.1, 176.1, 160.6, 148.8, 143.5, 142.5, 137.8, 136.2, 130.8, 130.0, 129.0, 128.5, 127.9, 127.7, 127.2, 126.0, 124.1, 123.4, 122.8, 122.2, 118.2, 116.1, 110.2, 110.0, 79.7, 75.1, 73.3, 43.6; HRMS (ESI) calculated for C35H28N5O2 (M + H+): 550.22375, found 550.22229.
1; 1H NMR (400 MHz, DMSO): δ 10.45 (s, 1H), 8.59 (d, J = 4.0 Hz, 1H), 8.50 (d, J = 8.0 Hz, 1H), 7.81 (t, J = 7.2 Hz, 1H), 7.46 (d, J = 7.2 Hz, 1H), 7.33 (t, J = 5.6 Hz, 1H), 7.25–7.21 (m, 4H), 7.14–7.07 (m, 3H), 6.98 (d, J = 7.6 Hz, 1H), 6.88–6.71 (m, 6H), 6.65 (d, J = 7.6 Hz, 1H), 6.43 (t, J = 6.8 Hz, 1H), 6.10 (d, J = 6.8 Hz, 2H), 4.93 (d, J = 15.6 Hz, 1H), 4.86 (d, J = 15.6 Hz, 1H), 4.35 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 177.1, 176.1, 160.6, 148.8, 143.5, 142.5, 137.8, 136.2, 130.8, 130.0, 129.0, 128.5, 127.9, 127.7, 127.2, 126.0, 124.1, 123.4, 122.8, 122.2, 118.2, 116.1, 110.2, 110.0, 79.7, 75.1, 73.3, 43.6; HRMS (ESI) calculated for C35H28N5O2 (M + H+): 550.22375, found 550.22229.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.26 (s, 1H), 8.59 (d, J = 4.4 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.34–7.21 (m, 5H), 7.12 (t, J = 8.0 Hz, 1H), 7.05 (d, J = 7.6 Hz, 1H), 6.99 (d, J = 7.2 Hz, 3H), 6.82 (t, J = 7.2 Hz, 1H), 6.77–6.72 (m, 3H), 6.67 (d, J = 7.6 Hz, 1H), 6.55 (d, J = 7.6 Hz, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.02 (d, J = 7.2 Hz, 2H), 5.04 (d, J = 15.6 Hz, 1H), 4.81 (d, J = 15.6 Hz, 1H), 4.27 (d, J = 9.2 Hz, 1H), 2.08 (s, 3H); 13C NMR (100 MHz, DMSO): δ 177.2, 176.1, 160.7, 148.8, 143.7, 142.6, 141.0, 137.7, 136.2, 131.2, 131.0, 129.0, 128.5, 127.8, 127.3, 127.2, 126.8, 124.1, 123.5, 123.4, 122.8, 122.7, 118.1, 116.1, 110.2, 109.8, 79.7, 75.1, 73.4, 43.5, 21.1; HRMS (ESI) calculated for C36H30N5O2 (M + H+): 564.23940, found 564.23816.
1; 1H NMR (400 MHz, DMSO): δ 10.26 (s, 1H), 8.59 (d, J = 4.4 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.34–7.21 (m, 5H), 7.12 (t, J = 8.0 Hz, 1H), 7.05 (d, J = 7.6 Hz, 1H), 6.99 (d, J = 7.2 Hz, 3H), 6.82 (t, J = 7.2 Hz, 1H), 6.77–6.72 (m, 3H), 6.67 (d, J = 7.6 Hz, 1H), 6.55 (d, J = 7.6 Hz, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.02 (d, J = 7.2 Hz, 2H), 5.04 (d, J = 15.6 Hz, 1H), 4.81 (d, J = 15.6 Hz, 1H), 4.27 (d, J = 9.2 Hz, 1H), 2.08 (s, 3H); 13C NMR (100 MHz, DMSO): δ 177.2, 176.1, 160.7, 148.8, 143.7, 142.6, 141.0, 137.7, 136.2, 131.2, 131.0, 129.0, 128.5, 127.8, 127.3, 127.2, 126.8, 124.1, 123.5, 123.4, 122.8, 122.7, 118.1, 116.1, 110.2, 109.8, 79.7, 75.1, 73.4, 43.5, 21.1; HRMS (ESI) calculated for C36H30N5O2 (M + H+): 564.23940, found 564.23816.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.23 (s, 1H), 8.59 (d, J = 4.0 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 7.81 (t, J = 7.6 Hz, 1H), 7.33 (t, J = 5.6 Hz, 1H), 7.24 (s, 3H), 7.14–7.10 (m, 2H), 7.00 (d, J = 7.2 Hz, 3H), 6.83–6.68 (m, 6H), 6.58 (d, J = 8.8 Hz, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.01 (d, J = 6.4 Hz, 2H), 5.06 (d, J = 16.0 Hz, 1H), 4.83 (d, J = 16.0 Hz, 1H), 4.38 (d, J = 8.8 Hz, 1H), 3.55 (s, 3H); 13C NMR (100 MHz, DMSO): δ 177.1, 176.1, 160.6, 155.2, 148.7, 143.6, 142.6, 137.8, 136.5, 136.1, 130.1, 129.0, 128.5, 127.8, 127.4, 127.3, 124.4, 124.1, 123.4, 122.8, 118.2, 116.1, 116.0, 112.9, 110.5, 110.2, 79.8, 75.4, 73.4, 55.7, 43.5; HRMS (ESI) calculated for C36H30N5O3 (M + H+): 580.23432, found 580.23254.
1; 1H NMR (400 MHz, DMSO): δ 10.23 (s, 1H), 8.59 (d, J = 4.0 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 7.81 (t, J = 7.6 Hz, 1H), 7.33 (t, J = 5.6 Hz, 1H), 7.24 (s, 3H), 7.14–7.10 (m, 2H), 7.00 (d, J = 7.2 Hz, 3H), 6.83–6.68 (m, 6H), 6.58 (d, J = 8.8 Hz, 1H), 6.43 (t, J = 7.2 Hz, 1H), 6.01 (d, J = 6.4 Hz, 2H), 5.06 (d, J = 16.0 Hz, 1H), 4.83 (d, J = 16.0 Hz, 1H), 4.38 (d, J = 8.8 Hz, 1H), 3.55 (s, 3H); 13C NMR (100 MHz, DMSO): δ 177.1, 176.1, 160.6, 155.2, 148.7, 143.6, 142.6, 137.8, 136.5, 136.1, 130.1, 129.0, 128.5, 127.8, 127.4, 127.3, 124.4, 124.1, 123.4, 122.8, 118.2, 116.1, 116.0, 112.9, 110.5, 110.2, 79.8, 75.4, 73.4, 55.7, 43.5; HRMS (ESI) calculated for C36H30N5O3 (M + H+): 580.23432, found 580.23254.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.60 (s, 1H), 8.59 (d, J = 4.8 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.82 (t, J = 7.2 Hz, 1H), 7.44 (d, J = 7.6 Hz, 1H), 7.36–7.33 (m, 1H), 7.27–7.22 (m, 5H), 7.04–7.01 (m, 2H), 6.93 (s, 1H), 6.89 (t, J = 10.4 Hz, 1H), 6.86–6.74 (m, 3H), 6.69 (t, J = 9.6 Hz, 2H), 6.48 (t, J = 7.6 Hz, 1H), 6.02 (d, J = 7.2 Hz, 2H), 4.93 (d, J = 15.6 Hz, 1H), 4.85 (d, J = 15.6 Hz, 1H), 4.48 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.7, 176.2, 160.3, 148.7, 143.4, 142.5, 142.3, 137.8, 135.8, 131.0, 130.0, 129.1, 128.7, 128.0, 127.7, 126.9, 126.8, 126.2, 125.1, 124.1, 123.4, 123.1, 122.4, 118.6, 116.2, 111.7, 110.2, 79.7, 75.4, 73.2, 43.7; HRMS (ESI) calculated for C35H28ClN5O2 (M + H+): 584.18478, found 584.18414.
1; 1H NMR (400 MHz, DMSO): δ 10.60 (s, 1H), 8.59 (d, J = 4.8 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.82 (t, J = 7.2 Hz, 1H), 7.44 (d, J = 7.6 Hz, 1H), 7.36–7.33 (m, 1H), 7.27–7.22 (m, 5H), 7.04–7.01 (m, 2H), 6.93 (s, 1H), 6.89 (t, J = 10.4 Hz, 1H), 6.86–6.74 (m, 3H), 6.69 (t, J = 9.6 Hz, 2H), 6.48 (t, J = 7.6 Hz, 1H), 6.02 (d, J = 7.2 Hz, 2H), 4.93 (d, J = 15.6 Hz, 1H), 4.85 (d, J = 15.6 Hz, 1H), 4.48 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.7, 176.2, 160.3, 148.7, 143.4, 142.5, 142.3, 137.8, 135.8, 131.0, 130.0, 129.1, 128.7, 128.0, 127.7, 126.9, 126.8, 126.2, 125.1, 124.1, 123.4, 123.1, 122.4, 118.6, 116.2, 111.7, 110.2, 79.7, 75.4, 73.2, 43.7; HRMS (ESI) calculated for C35H28ClN5O2 (M + H+): 584.18478, found 584.18414.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.58 (s, 1H), 8.59 (d, J = 4.0 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.82 (t, J = 7.2 Hz, 1H), 7.44 (d, J = 7.2 Hz, 1H), 7.36–7.32 (m, 2H), 7.25 (s, 4H), 7.05–7.02 (m, 3H), 6.87 (t, J = 7.6 Hz, 1H), 6.80 (t, J = 7.6 Hz, 2H), 6.71–6.67 (m, 3H), 6.48 (t, J = 6.8 Hz, 1H), 6.01 (d, J = 5.6 Hz, 2H), 4.92 (d, J = 15.6 Hz, 1H), 4.85 (d, J = 15.6 Hz, 1H), 4.48 (d, J = 8.8 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.6, 176.2, 160.3, 148.7, 143.4, 142.9, 142.2, 137.8, 135.8, 132.8, 131.0, 130.2, 129.5, 129.2, 129.1, 128.7, 128.0, 127.8, 127.7, 126.2, 125.4, 124.1, 123.4, 123.1, 122.4, 118.6, 117.6, 116.1, 114.7, 112.2, 110.2, 79.7, 75.4, 73.1, 43.6; HRMS (ESI) calculated for C35H28BrN5O2 (M + H+): 628.13426, found 628.13348.
1; 1H NMR (400 MHz, DMSO): δ 10.58 (s, 1H), 8.59 (d, J = 4.0 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.82 (t, J = 7.2 Hz, 1H), 7.44 (d, J = 7.2 Hz, 1H), 7.36–7.32 (m, 2H), 7.25 (s, 4H), 7.05–7.02 (m, 3H), 6.87 (t, J = 7.6 Hz, 1H), 6.80 (t, J = 7.6 Hz, 2H), 6.71–6.67 (m, 3H), 6.48 (t, J = 6.8 Hz, 1H), 6.01 (d, J = 5.6 Hz, 2H), 4.92 (d, J = 15.6 Hz, 1H), 4.85 (d, J = 15.6 Hz, 1H), 4.48 (d, J = 8.8 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 176.6, 176.2, 160.3, 148.7, 143.4, 142.9, 142.2, 137.8, 135.8, 132.8, 131.0, 130.2, 129.5, 129.2, 129.1, 128.7, 128.0, 127.8, 127.7, 126.2, 125.4, 124.1, 123.4, 123.1, 122.4, 118.6, 117.6, 116.1, 114.7, 112.2, 110.2, 79.7, 75.4, 73.1, 43.6; HRMS (ESI) calculated for C35H28BrN5O2 (M + H+): 628.13426, found 628.13348.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.51 (s, 1H), 8.59 (d, J = 4.0 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 8.0 Hz, 1H), 7.45 (d, J = 7.6 Hz, 1H), 7.32 (t, J = 5.6 Hz, 1H), 7.25–7.20 (m, 4H), 7.07 (d, J = 2.8 Hz, 2H), 6.93 (d, J = 7.6 Hz, 1H), 6.88–6.84 (m, 2H), 6.77–6.71 (m, 3H), 6.66–6.61 (m, 2H), 6.43 (t, J = 7.2 Hz, 1H), 6.00 (d, J = 7.2 Hz, 2H), 4.87 (s, 2H), 4.30 (d, J = 9.2 Hz, 1H), 2.03 (s, 3H); 13C NMR (100 MHz, DMSO): δ 177.0, 176.2, 160.7, 148.7, 143.5, 142.5, 141.2, 137.8, 136.3, 131.4, 130.8, 130.4, 129.0, 128.5, 127.9, 127.8, 127.6, 126.0, 124.1, 123.4, 122.2, 118.0, 115.9, 110.0, 79.8, 75.1, 73.2, 43.6, 21.2; HRMS (ESI) calculated for C36H30N5O2 (M + H+): 564.23940, found 564.23840.
1; 1H NMR (400 MHz, DMSO): δ 10.51 (s, 1H), 8.59 (d, J = 4.0 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 8.0 Hz, 1H), 7.45 (d, J = 7.6 Hz, 1H), 7.32 (t, J = 5.6 Hz, 1H), 7.25–7.20 (m, 4H), 7.07 (d, J = 2.8 Hz, 2H), 6.93 (d, J = 7.6 Hz, 1H), 6.88–6.84 (m, 2H), 6.77–6.71 (m, 3H), 6.66–6.61 (m, 2H), 6.43 (t, J = 7.2 Hz, 1H), 6.00 (d, J = 7.2 Hz, 2H), 4.87 (s, 2H), 4.30 (d, J = 9.2 Hz, 1H), 2.03 (s, 3H); 13C NMR (100 MHz, DMSO): δ 177.0, 176.2, 160.7, 148.7, 143.5, 142.5, 141.2, 137.8, 136.3, 131.4, 130.8, 130.4, 129.0, 128.5, 127.9, 127.8, 127.6, 126.0, 124.1, 123.4, 122.2, 118.0, 115.9, 110.0, 79.8, 75.1, 73.2, 43.6, 21.2; HRMS (ESI) calculated for C36H30N5O2 (M + H+): 564.23940, found 564.23840.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1H NMR (400 MHz, DMSO): δ 10.50 (s, 1H), 8.59 (d, J = 4.4 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.46 (d, J = 7.6 Hz, 1H), 7.34–7.21 (m, 5H), 7.07 (d, J = 2.8 Hz, 2H), 6.87 (d, J = 7.6 Hz, 1H), 6.78–6.60 (m, 7H), 6.45 (t, J = 7.2 Hz, 1H), 6.03 (d, J = 7.2 Hz, 2H), 4.89 (d, J = 15.6 Hz, 1H), 4.84 (d, J = 15.6 Hz, 1H), 4.31 (d, J = 9.2 Hz, 1H), 3.51 (s, 3H); 13C NMR (100 MHz, DMSO): δ 176.7, 176.1, 160.6, 155.3, 148.8, 143.5, 142.5, 137.8, 136.7, 136.3, 130.8, 129.0, 128.6, 127.9, 127.8, 126.1, 124.3, 124.1, 123.4, 123.3, 122.2, 118.2, 116.1, 115.0, 113.6, 110.5, 110.0, 79.7, 75.3, 73.3, 55.7, 43.6; HRMS (ESI) calculated for C36H30N5O3 (M + H+): 580.23432, found 580.23279.
1; 1H NMR (400 MHz, DMSO): δ 10.50 (s, 1H), 8.59 (d, J = 4.4 Hz, 1H), 8.49 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.46 (d, J = 7.6 Hz, 1H), 7.34–7.21 (m, 5H), 7.07 (d, J = 2.8 Hz, 2H), 6.87 (d, J = 7.6 Hz, 1H), 6.78–6.60 (m, 7H), 6.45 (t, J = 7.2 Hz, 1H), 6.03 (d, J = 7.2 Hz, 2H), 4.89 (d, J = 15.6 Hz, 1H), 4.84 (d, J = 15.6 Hz, 1H), 4.31 (d, J = 9.2 Hz, 1H), 3.51 (s, 3H); 13C NMR (100 MHz, DMSO): δ 176.7, 176.1, 160.6, 155.3, 148.8, 143.5, 142.5, 137.8, 136.7, 136.3, 130.8, 129.0, 128.6, 127.9, 127.8, 126.1, 124.3, 124.1, 123.4, 123.3, 122.2, 118.2, 116.1, 115.0, 113.6, 110.5, 110.0, 79.7, 75.3, 73.3, 55.7, 43.6; HRMS (ESI) calculated for C36H30N5O3 (M + H+): 580.23432, found 580.23279.Acknowledgements
We thank Beijing Municipal Commission of Education (No. JC015001200902), Beijing Municipal Natural Science Foundation (No. 7102010, No. 2122008), Basic Research Foundation of Beijing University of Technology (X4015001201101), Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (No. PHR201008025), Doctoral Scientific Research Start-up Foundation of Beijing University of Technology (No. 52015001200701) for financial supports.Notes and references
- (a) R. Murugan, S. Anbazhagan, Lingeshwaran and S. Sriman Narayanan, Eur. J. Med. Chem., 2009, 44, 3272–3279 CrossRef CAS PubMed; (b) A. S. Girgis, Eur. J. Med. Chem., 2009, 44, 1257–1264 CrossRef CAS PubMed; (c) P. Prasanna, K. Balamurugan, S. Perumal, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 5653–5661 CrossRef CAS PubMed; (d) K. Karthikeyan, P. M. Sivakumar, M. Doble and P. T. Perumal, Eur. J. Med. Chem., 2010, 45, 3446–3452 CrossRef CAS PubMed; (e) Y. Arun, G. Bhaskar, C. Balachandran, S. Ignacimuthu and P. T. Perumal, Bioorg. Med. Chem. Lett., 2013, 23, 1839–1845 CrossRef CAS PubMed.
- (a) R. S. Kumar, S. M. Rajesh, S. Perumal, D. Banerjee, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 411–422 CrossRef CAS PubMed; (b) B. V. S. Reddy, G. Karthik, T. Rajasekaran and B. Sridhar, Eur. J. Org. Chem., 2015, 2038–2041 CrossRef; (c) F. M. Moghaddam, M. Kiamehra, M. R. Khodabakhshia, M. J. Javana, S. Fathia, A. Villingerb, V. O. Iaroshenko and P. Langer, Helv. Chim. Acta, 2013, 96, 2103–2114 CrossRef; (d) G.-L. Shen, J. Sun and C.-G. Yan, RSC Adv., 2015, 5, 4475–4483 RSC; (e) L. Wang, Y. Zhang, H.-Y. Hu, H. K. Fun and J.-H. Xu, J. Org. Chem., 2005, 70, 3850–3858 CrossRef CAS PubMed; (f) J. Sun, Y. J. Xie and C. G. Yan, J. Org. Chem., 2013, 78, 8354–8365 CrossRef CAS PubMed; (g) K. Suman, L. Srinu and S. Thennarasu, Org. Lett., 2014, 16, 3732–3735 CrossRef CAS PubMed; (h) S. U. Maheswari, S. Perumal and A. I. Almansour, Tetrahedron Lett., 2012, 53, 349–353 CrossRef CAS; (i) Y. Hu, Y. Zou, H. Wu and D. Shi, Ultrason. Sonochem., 2012, 19, 264–269 CrossRef CAS PubMed; (j) J. He, G. Ouyang, Z. Yuan, R. Tong, J. Shi and L. Ouyang, Molecules, 2013, 18, 5142–5154 CrossRef CAS PubMed; (k) Z. Huang, Q. Zhao, G. Chen, H. Wang, W. Lin, L. Xu, H. Liu, J. Wang, D. Shi and Y. Wang, Molecules, 2012, 17, 12704–12717 CrossRef CAS PubMed; (l) R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, J. Med. Chem., 2008, 51, 5731–5735 CrossRef CAS PubMed; (m) A. Kaur, M. Kaur and B. Singh, J. Heterocycl. Chem., 2015, 52, 827–833 CrossRef CAS.
- (a) Y. A. Ivanenkov, S. V. Vasilevski, E. K. Beloglazkina, M. E. Kukushkin, A. E. Machulkin, M. S. Veselov, N. V. Chufarova, E. S. Chernyaginab, A. S. Vanzcool, N. V. Zyk, D. A. Skvortsov, A. A. Khutornenko, A. L. Rusanov, A. G. Tonevitsky, O. A. Dontsova and A. G. Majouga, Bioorg. Med. Chem. Lett., 2015, 25, 404–409 CrossRef CAS PubMed; (b) D. Kathirvelan, J. Haribabu, B. S. R. Reddy, C. Balachandran and V. Duraipandiyan, Bioorg. Med. Chem. Lett., 2015, 25, 389–399 CrossRef CAS PubMed; (c) H. Dong, S. Song, J. Li, C. Xu, H. Zhang and L. Ouyang, Bioorg. Med. Chem. Lett., 2015, 25, 3585–3591 CrossRef CAS PubMed; (d) J. R. McConnell, L. K. Buckton and S. R. McAlpine, Bioorg. Med. Chem. Lett., 2015, 25, 3391–3407 CrossRef PubMed.
- (a) A. S. Girgis, Eur. J. Med. Chem., 2009, 44, 91–100 CrossRef CAS PubMed; (b) A. I. Almansour, S. Ali, M. A. Ali, R. Ismail, T. S. Choon, V. Sellappan, K. K. Elumalai and S. Pandian, Bioorg. Med. Chem. Lett., 2012, 22, 7418–7421 CrossRef CAS PubMed; (c) C. Mhiri, S. Boudriga, M. Askri, M. Knorr, D. Sriram, P. Yogeeswari, F. Nana, C. Golz and C. Strohmann, Bioorg. Med. Chem. Lett., 2015, 25, 4308–4313 CrossRef CAS PubMed; (d) B. Yu, D. Q. Yu and H. M. Liu, Eur. J. Med. Chem., 2015, 97, 673–698 CrossRef CAS PubMed; (e) D. Chakraborty, A. Maity, C. K. Jain, A. Hazra, Y. P. Bharitkar, T. Jha, H. K. Majumder, S. Roychoudhury and N. B. Mondal, Med. Chem. Commun., 2015, 6, 702–707 RSC; (f) J. A. Xiao, H. G. Zhang, S. Liang, J. W. Ren, H. Yang and X. Q. Chen, J. Org. Chem., 2013, 78, 11577–11583 CrossRef CAS PubMed; (g) A. I. Almansour, N. Arumugam, R. S. Kumar, G. Periyasami, H. A. Ghabbour and H. K. Fun, Molecules, 2015, 20, 780–791 CrossRef PubMed.
- Y. Y. Han, W. B. Chen, W. Y. Han, Z. J. Wu, X. M. Zhang and W. C. Yuan, Org. Lett., 2012, 14, 490–493 CrossRef CAS PubMed.
- P. Drouhin, T. E. Hurst, A. C. Whitwood and R. J. Taylor, Org. Lett., 2014, 16, 4900–4903 CrossRef CAS PubMed.
- Y. H. Sun, Y. Xiong, C. Q. Peng, W. Li, J. A. Xiao and H. Yang, Org. Biomol. Chem., 2015, 13, 7907–7910 CAS.
- K. Suman and S. Thennarasu, RSC Adv., 2015, 5, 79413–79422 RSC.
- CCDC 1054674 contains the supplementary crystallographic data for compound 4de†.
- (a) C. Janiak, J. Chem. Soc., Dalton Trans., 2000, 21, 3885–3896 RSC; (b) M. O. Sinnokrot, E. F. Valeev and C. David Sherrill, J. Am. Chem. Soc., 2002, 124, 10887–10893 CrossRef CAS PubMed.
- ESI†.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of NMR for imidazolidine-dispirooxindoles 4; X-ray single crystal structure analysis data for 4de.11 CCDC 1054674. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra21995g |
| This journal is © The Royal Society of Chemistry 2015 |