Indium recovery from waste liquid crystal displays by polyvinyl chloride waste
Abstract
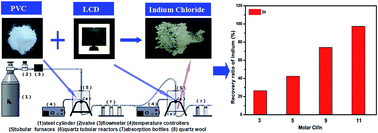
Indium (In), a rare element existing only in a few natural minerals, is mainly used to produce indium tin oxide (ITO) thin films for applications including liquid crystal displays (LCDs). Therefore, developing an effective method to recover In from waste LCDs is of great significance. In the present study, In was extracted from indium oxide (In2O3) and LCD powder after alkali dissolution via a chloride volatilization process using polyvinyl chloride (PVC) as the chlorination precursor. High purity In2O3 was investigated firstly in a nitrogen atmosphere. The results indicated that high purity In2O3 could have a high recovery ratio of indium using polyvinyl chloride (PVC) as the chlorination agent. The recovery ratio of indium could reach 99.36%. The product was analyzed by X-ray diffraction (XRD), and the results indicated that the condensed products were InCl3 and InCl. Real LCD powder after alkali dissolution was also examined. The recovery ratio of indium was 97.50% and the utilization of hydrogen chloride was 53.91% under the conditions of a molar Cl/In ratio of 11, a degradation temperature of PVC of 400 °C, a chlorination temperature of 500 °C, carrier gas flow rate at 0.1 L min−1, and a reaction time of 30 min. The condensed products were also mainly InCl3 and InCl. It is an effective method to recover In from LCD panels.

 Please wait while we load your content...
Please wait while we load your content...