A smart rhodamine–pyridine conjugate for bioimaging of thiocyanate in living cells†
Sandip Mandala,
Animesh Sahanaa,
Arnab Banerjeea,
Damir A. Safin*b,
Maria G. Babashkinab,
Koen Robeynsb,
Sjoerd Verkaartc,
Joost G. J. Hoenderopc,
Mariusz P. Mitoraj*d,
Yann Garcia*b and
Debasis Das*a
aDepartment of Chemistry, The University of Burdwan, 713104, Burdwan, West Bengal, India. E-mail: ddas100in@yahoo.com; Fax: +91 342 2530452
bInstitute of Condensed Matter and Nanosciences, Molecules, Solids and Reactivity (IMCN/MOST), Université catholique de Louvain, Place L. Pasteur 1, 1348 Louvain-la-Neuve, Belgium. E-mail: damir.a.safin@gmail.com; yann.garcia@uclouvain.be; Fax: +32 1047 2330; Tel: +32 1047 2831
cDepartment of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, 6500 HB Nijmegen, The Netherlands
dDepartment of Theoretical Chemistry, Faculty of Chemistry, Jagiellonian University, R. Ingardena 3, 30-060 Cracow, Poland. E-mail: mitoraj@chemia.uj.edu.pl
First published on 24th November 2015
Abstract
A rhodamine–pyridine conjugate, REDA-2PC, can selectively detect NCS− in human embryonic kidney 293 (HEK293) cells. DFT calculations suggest that this is due to non-covalent interactions (hydrogen bonding N–H⋯NCS−, π–π stacking) and long range electrostatic forces acting between the sulfur atom of the NCS− anion and the NEt2 unit of REDA-2PC. A visible light excitable probe allows fluorescence and naked eye detection of nanomolar NCS−, much lower than normal NCS− levels in the human body. A “lock” and “key” sensing mechanism is established and compared with the corresponding fluorescein derivative as a model compound.
Introduction
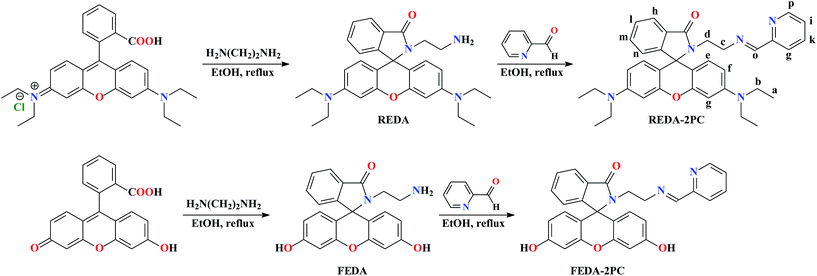
Sickle cell disease, characterized by crescent-shaped or sickled red blood cells and chronic anemia, is caused by abnormally high destruction of red blood cells. Thiocyanate (NCS−) is known to reduce the sickling of red blood cells and completely prevent sickling in many individuals.1 NCS− binding to amino terminal valine residues of haemoglobin is called carbamylation.2 Cystic fibrosis (CF), a pleiotropic/genetic disease is caused by mutations of gene for the cystic fibrosis transmembrane conductance regulator (CFTCR) protein.3 Patients having mutations in CFTCR are immune compromised as they lack NCS− transport into the extracellular milieu. However, this process has not been visualized and therefore a NCS− sensor/probe for live cell imaging is warranted.Recently, we have reported the fluorescent probe, named rhodamine–thiophene conjugate RTA, to detect intra-cellular NCS−, and to determine concentration of NCS− in blood serum and squamous epithelium cells (before and after smoking).4 The detection limit of RTA was found to be 10−8 M, which is much lower than that for the record limit 1.51 × 10−5 M.5 Now we have used RTA to detect NCS− in the human embryonic kidney 293 (HEK293) cells. It was observed that after a certain time the fluorescence intensity of the cells reduced due to diffusion of the [RTA + NCS−] complex across the cellular membrane into extracellular medium. In the presence of NCS−, the spirolactam ring opens to the amide form. As amides are good cell permeable agents,6 they can easily exit cells reducing intracellular fluorescence signal. Our aim was to design and develop new probe to allow the intra-cellular fluorescence signal intact for a long time. The present probe, a rhodamine–pyridine conjugate, (E)-3′,6′-bis(diethylamino)-2-(2-(pyridin-2-ylmethyleneamino)ethyl)spiro[isoindoline-1,9′-xanthen]-3-one (REDA-2PC) acts first via a “lock” and “key” mechanism and, subsequently, undergoes hydrolysis to amine having less cell permeability than other derivatives like imine or amide due to higher polarity,7–9 and gets trapped inside the cell. Presence of the imine bond appended to the pyridine moiety allows facile hydrolysis of the probe, REDA-2PC.
Results and discussion
REDA-2PC has been synthesised by reacting rhodamine B with ethylenediamine followed by condensation with 2-pyridinecarboxaldehyde (Scheme 1), and characterized by 1H NMR, FTIR and mass spectroscopy (Fig. S1–S3 in the ESI†).Efficiency of a fluorescence probe highly depends on pH of the medium. Thus, optimization of pH is very essential. REDA-2PC and NCS−, mixed in different sets of pH (Fig. S4 in the ESI†), show maximum emission intensity in the pH range 4.0–7.5, while the fluorescence intensity of free REDA-2PC is maximum at pH 4.0 and minimum at pH 7.0–8.0. Emission of REDA-2PC at 571 nm is very weak due to the closed spirolactam ring with a quantum yield of 0.03 (Fig. S5 in the ESI†).
About 26 fold fluorescence enhancement at 580 nm (∼15 times increase in the fluorescence quantum yield to 0.44) occurs upon addition of NCS− due to a spirolactam ring opening. Emission properties of REDA-2PC in the presence of different anions have also been investigated. Negligible fluorescence enhancement is observed in the presence of ClO4− (Fig. S5 in the ESI†). UV light exposed color of REDA-2PC in the presence of different anions is shown in Fig. S6 in the ESI.†
Gradual addition of NCS− to REDA-2PC enhances the emission intensity gradually (Fig. 1). Associated UV light exposed color changes are shown in Fig. S7 in the ESI.† Plot of emission intensities of REDA-2PC as a function of added NCS− generates a sigmoidal curve (Fig. S8 in the ESI†), whose linear regression (up to 10 μM of NCS−) is useful to determine an unknown concentration of NCS−.
Effect of different anions on the absorption properties of REDA-2PC has been investigated in the same media (Fig. S9 in the ESI†). The absorption spectrum of the probe reveals a drastic change in the presence of NCS−, while other anions have no influence. Naked eye color of REDA-2PC in the presence of these anions is shown in Fig. S10 in the ESI.† REDA-2PC has a very weak absorbance at 543 nm, characteristics for the closed spirolactam ring. Gradual addition of NCS− has red-shifted the peak to 554 nm with the appearance of a pink color, while the absorbance enhanced ∼100 times due to a spirolactam ring opening (Fig. 1). Gradual changes in its color intensity with added NCS− are shown in Fig. S11 in the ESI.† Plot of absorbance of REDA-2PC vs. concentration of NCS− results a sigmoidal curve. The linear region exists up to 10 μM NCS−, which is useful for the determination of unknown concentration of NCS− (Fig. S12 in the ESI†). No significant interference from different cations and anions has been found for the emission properties of NCS−-added REDA-2PC (Fig. S13 in the ESI†). Naked eye and UV light exposed views of the [REDA-2PC + NCS−] system in the presence of ions are shown in Fig. S14 in the ESI.† The binding constant of REDA-2PC with NCS− has been estimated, using the Benesi–Hildebrand equation,10 as 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 (Fig. S15 in the ESI†). The reaction stoichiometry has been found as REDA-2PC
800 M−1 (Fig. S15 in the ESI†). The reaction stoichiometry has been found as REDA-2PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCS− = 1
NCS− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S16 in the ESI†).
1 (Fig. S16 in the ESI†).
1H NMR titration reveals drastic changes of the spectra of REDA-2PC upon gradual addition of NCS−. Two new peaks at 8.75 ppm and 9.98 ppm, corresponding to the NH proton due to a spirolactam ring opening and aldehyde proton of pyridine-2-carboxaldehyde, generated due to hydrolysis of the imine bond, have been clearly observed (Fig. S1 in the ESI†). This fact supports that the N-center of one of the NEt2 groups becomes positively charged and stabilised by close association with the NCS− anion. This is also corroborated from comparison of their FTIR spectra. The carbonyl stretching of the free probe at 1686 cm−1 has been shifted to 1615 cm−1 upon interacting with NCS− (Fig. S2 in the ESI†). Appearance of a new stretching peak for NCS− at 2065 cm−1 also supports the mechanism.
To further strengthen the binding mechanism, a fluorescein based model compound FEDA-2PC has been synthesised (Scheme 1). FEDA is characterized by single crystal X-ray diffraction (Fig. 2, Tables S1 and S2 in the ESI†), while FEDA-2PC is characterized by 1H NMR (Fig. S17 in the ESI†) and QTOF-mass spectroscopy (Fig. S18 in the ESI†). In FEDA-2PC, two OH groups are present instead of two NEt2 groups in REDA-2PC. This model compound is insensitive towards NCS− which is quite reasonable, as there is no scope for NCS− to become attached to FEDA-2PC and, consequently, the spirolactam ring remains intact, strongly supporting our proposed mechanism. Emission intensity of free FEDA-2PC at 465 nm remains almost unchanged after addition of NCS− up to 500 μM (Fig. S19 in the ESI†) under the same experimental conditions used for REDA-2PC. Thus, NCS− assisted strong fluorescence enhancement of REDA-2PC (Fig. 3) is attributed to the spirolactam ring opening followed by hydrolysis to free rhodamine–ethylenediamine and pyridine-2-carboxaldehyde, also evident from the mass spectrum (Fig. S20 in the ESI†).
It was found that REDA-2PC is a highly efficient probe to monitor intracellular NCS− concentration in HEK293 cells. In particular, HEK293 cells, seeded on fibronectin-coated glass coverslips, were preloaded with REDA-2PC in the absence or presence of NCS−. After 15 min cells were washed, transferred to an incubation chamber containing buffer and placed on the stage of a fluorescent microscope after which images were captured and intensities were calculated (Fig. S21 in the ESI†). REDA-2PC fluorescence was significantly higher in the NCS− treated HEK293 cells over a wide range of NCS− concentrations compared to untreated control ones (Fig. 4) without affecting cell viability (Fig. S22 in the ESI†). Additionally, intracellular REDA-2PC fluorescence in HEK293 cells responded to acute administration of NCS− (100 μM, final concentration) resulting increased fluorescence whereas this effect was less pronounced in the RTA-loaded HEK293 cells (Fig. S23 in the ESI†). Dilution of the extracellular RTA or REDA-2PC concentration by addition of CaHT resulted in a rapid decrease in intracellular fluorescence, which was more pronounced in the RTA than in the REDA-2PC-treated cells, suggesting an improved intracellular retention of REDA-2PC (Fig. 5). Additionally, enhancement of fluorescence intensity upon addition of NCS− is higher for REDA-2PC than RTA (Fig. S24 in the ESI†). Effect of the solvent composition on the emission intensities of REDA-2PC in the absence and presence of NCS− is shown in Fig. S25 in the ESI.†
 | ||
| Fig. 4 Fluorescence microscope images of REDA-2PC treated HEK293 cells in the absence and presence of NCS−. | ||
 | ||
| Fig. 5 Effect of the NCS− addition to RTA (5 μM) and REDA-2PC (5 μM) loaded cells after washing with CaHT (500 μM) as control. | ||
REDA-2PC can also determine NCS− concentration in living cells and biological samples viz. sheep blood serum (81.20 ± 0.01 μM, Table S3 in the ESI†) and cow milk (1.31 ± 0.02 mM, Table S4 in the ESI†).
In order to shed light on the interaction between NCS− and REDA-2PC, static DFT calculations based on the ADF program11 with DFT/BLYP-D3/TZP as well as ab initio Car–Parrinello molecular dynamics simulations by means of the CPMD package were performed.12
The lowest energy ground state conformations of the neutral REDA-2PC, its protonated form [REDA-2PCH]+ and the corresponding complexes with NCS− were first determined (Fig. 6). The cationic models were also considered since hydrolysis of the imine bond leads to the formation of the NH unit due to a spirolactam ring opening.
It is clearly seen from [REDA-2PCH]+⋯NCS− that the NCS− sensing can originate from non-covalent interactions. In particular, NCS− forms both hydrogen bonding of the N–H⋯NCS− type with the NH fragment as well as π–π stacking interactions with the C6H3 ring of the rhodamine plane in [REDA-2PCH]+. In order to include entropic effects and to understand the behaviour of the [REDA-2PCH]+⋯NCS− complex at more realistic conditions, ab initio Car–Parrinello molecular dynamics simulations were further carried out (at room temperature of 298.15 K). It is established that NCS− is continuously interacting via N–H⋯NCS− bonding with [REDA-2PCH]+ within the overall simulation time of ∼11 ps (see animation [reda-2pch]-ncs.wmv in the ESI†). Furthermore, NCS− glides above the C6H3 plane back and forth, employing dynamically π–π stacking interactions. It is remarkable that the NEt2 fragment is tilted up towards the sulfur atom of NCS−, which suggests that they most likely additionally stabilize the system. It is evident from the molecular electrostatic potential (MEP) that the positively charged NEt2 hydrogen atoms (light green regions with the positive MEP values) can attract via long range electrostatic forces the negatively charged sulfur atom (red regions with the negative MEP values) of NCS− (Fig. S26 in the ESI†). These results explain qualitatively why the lack of sensing towards NCS− is observed when using FEDA-2PC, which contains OH units instead of bulky NEt2 fragments. In order to obtain deeper insight into the nature of bonding between NCS− and [REDA-2PCH]+ the charge and energy decomposition ETS-NOCV13 was performed (Fig. S27 in the ESI†). It is established, that the electrostatic contribution is the most important stabilizing factor (ΔEelstat = −79.68 kcal mol−1) followed by the orbital interaction (ΔEorb = −26.79 kcal mol−1) and the dispersion (ΔEdispersion = −12.11 kcal mol−1) term (Fig. S27 top in the ESI†). Further decomposition of ΔEorb into contributions (Fig. S27 bottom in the ESI†) demonstrate that the leading charge-transfer component, with the corresponding stabilization ΔEorb(N–H⋯NCS−) = −9.24 kcal mol−1, stems from the formation of hydrogen bonding. It is based on outflow of the electron density from the lone electron pair of the NCS− nitrogen atom to empty σ*(N–H). The second NOCV-based contribution, with the corresponding stabilization ΔEorb(π–π) = −6.02 kcal mol−1, demonstrate the change in densities of NCS− and, predominantly, the rhodamine plane of [REDA-2PCH]+ due to π–π stacking. It should be noted that both components involve charge outflow from the π-bonds of NCS−. This nicely explains the experimental lowering of the carbonyl stretching frequency (from 1686 cm−1 to 1615 cm−1) upon interaction with NCS−.
In order to obtain a qualitative picture of the absorption spectra we have performed a TD-DFT/B3LYP/TZP study14 with inclusion of solvent effects at the COSMO level15 as implemented in the ADF program for both (1)[REDA-2PCH]+⋯NCS−, where the spirolactam ring is opened, and neutral REDA-2PC, where the spirolactam ring is closed.
The dominant absorption band of (1)[REDA-2PCH]+⋯NCS− (f = 0.8378 a.u.) is observed at λ = 570.5 nm (Fig. 7). It is in fair agreement with the experimental value of 554 nm. Decomposition of this transition into the molecular orbitals shows that the absorption is of the π → π* type and it employs predominantly the HOMO orbitals located at the NCS− and REDA-2PCH]+ fragments, whereas the LUMO covers only the [REDA-2PCH]+ unit. It is important to note that when considering another isomer (2)[REDA-2PCH]+⋯NCS−, where NCS− is located at the outer region of the C6H3 ring (Fig. 6), the qualitative picture of absorption is rather similar. However, the oscillator strength of the dominant band becomes more pronounced f = 1.2521 a.u. and λ = 545.2 nm. Given the fact that in reality NCS− migrates in parallel above the rhodamine plane (see animation [reda-2pch]-ncs.wmv in the ESI†), one would have to average the absorption spectra in order to obtain more quantitatively exact characteristics. Nevertheless, TD-DFT results support qualitatively the experimental data as well as indirectly the proposed mechanism of binding of NCS−. Finally, it is important to state that when considering neutral REDA-2PC only one extremely weak band in the visible region at λ = 430.0 nm with the oscillator strength f = 0.0723 a.u. was noted from the TD-DFT calculations. It is in qualitative agreement with the experiment in a sense that the gradual addition of NCS− to REDA-2PC leads not only to the red-shift of the dominant band, but predominantly to significant amplification of the absorption intensity. The lack of quantitative agreement between our TD-DFT data with the experimental results might be related to various factors including the simplified “static” models considered in the TD-DFT calculations with respect to dynamic equilibria as well as approximations used within the TD-DFT approach.
The development of REDA-2PC as an intracellular NCS− probe pave the way for future diagnostic research in which NCS− levels are altered due to disease like in CF. As lung fluid and their accompanying immune cells are frequently used in diagnostic procedures in CF patients, one could envision the use of REDA-2PC to determine NCS− concentrations in lung fluid or immune cells of CF patients. Ideally, the amount of NCS− detected, as determined from the REDA-2PC fluorescence levels, could then be interpreted as a prognostic factor for immune function in CF patients. In addition, the development of the intracellular NCS− probe REDA-2PC allowed for the first time the possibility of identifying the molecular players involved in intracellular NCS− transport in living cells. One could envision to target human transcriptome using an siRNA library screen to specifically knock-down one known mRNA at the time. As REDA-2PC sensed changes in intracellular NCS− levels in HEK293 after NCS− addition (Fig. 4 and 5, and Fig. S21 and S23 in the ESI†), this change in fluorescence would be lost in HEK293 cells in which molecules involved in NCS− homeostasis were knocked-down, leading to the identification of the gene involved.
Conclusions
In summary, visible light excitable pyridine appended rhodamine derivative, REDA-2PC, selectively and efficiently detects NCS− in HEK293 cells. Theoretical modelling suggests that it can be due to non-covalent interactions (hydrogen bonding N–H⋯NCS−, π–π stacking) and long range electrostatic forces acting between the sulfur atom of the NCS− anion and the NEt2 unit of REDA-2PC. The probe allows fluorescence and naked eye detection of nanomolar NCS−, less than normal NCS− level in a human body. REDA-2PC has also been used to determine NCS− concentration in real samples such as sheep blood serum and cow milk. Additionally, the “lock” and “key” sensing mechanism is firmly established comparing with corresponding fluorescein derivative as a model compound.Experimental
Materials and methods
Rhodamine B, 2-pyridinecarboxaldehyde and ethylenediamine were purchased from Sigma Aldrich (India). NaNCS was purchased from Merck (India). Spectroscopic grade solvents have been used. Either Na+ or K+ salts of anions, and NO3− or Cl− salts of cations were used. Other chemicals are of analytical reagent grade and used without further purification. Milli-Q 18.2 MΩ cm−1 water has been used throughout all the experiments. A JASCO (model V-570) UV-vis spectrophotometer has been used for measuring the absorption spectra. FTIR spectra are recorded on a JASCO FTIR spectrometer (model: FTIR-H20). Mass spectra were obtained using QTOF Micro YA 263 mass spectrometer in ES positive mode. The electrospray ionization (ESI) mass spectra were measured with a Finnigan-Mat TCQ 700 mass spectrometer. The speed of sample submission was 2 μL min−1. The ionisation energy was 4.5 kV. The capillary temperature was 200 °C. 1H NMR spectra in CD3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O (9
D2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) have been recorded using Bruker Advance 300 (300 MHz) instrument. Steady-state fluorescence experiments are performed using Perkin-Elmer LS55 spectrofluorimeter. The emission from the sample was collected at a right angle to the direction of the excitation beam maintaining magic angle polarization (54.71). The full width at half maximum (FWHM) of the instrument response function was 250 ps and the resolution was 28 ps per channel. The data were fitted to multi exponential functions after deconvolution of the instrument response function by an iterative reconvolution technique using IBH DAS 6.2 data analysis software in which reduced w2 and weighted residuals serve as parameters for goodness of fit. pH measurements have been carried out on a Systronics digital pH meter (model 335, India). Elemental analysis has been made using a Perkin-Elmer CHN analyser with first 2000-analysis kit.
1, v/v) have been recorded using Bruker Advance 300 (300 MHz) instrument. Steady-state fluorescence experiments are performed using Perkin-Elmer LS55 spectrofluorimeter. The emission from the sample was collected at a right angle to the direction of the excitation beam maintaining magic angle polarization (54.71). The full width at half maximum (FWHM) of the instrument response function was 250 ps and the resolution was 28 ps per channel. The data were fitted to multi exponential functions after deconvolution of the instrument response function by an iterative reconvolution technique using IBH DAS 6.2 data analysis software in which reduced w2 and weighted residuals serve as parameters for goodness of fit. pH measurements have been carried out on a Systronics digital pH meter (model 335, India). Elemental analysis has been made using a Perkin-Elmer CHN analyser with first 2000-analysis kit.
Quantum yield measurements
Fluorescence quantum yields were determined using rhodamine B as a reference with a known ϕref value of 0.65 in basic EtOH.16 The area of the emission spectrum was integrated using the software available in the instrument and the quantum yield was calculated according to the following equation:17 ϕsample = ϕref × [Asample/Aref] × [ODref/ODsample] × [ηsample2/ηref2], where ϕsample and ϕref are the fluorescence quantum yield of the sample and reference, respectively; Asample and Aref are the area under the fluorescence spectra of the sample and the reference, respectively; ODsample and ODref are the corresponding optical densities of the sample and the reference solution at the wavelength of excitation; ηsample and ηref are the refractive index of the sample and reference, respectively.UV-vis and fluorescence titration
The path length of cells used for absorption and emission studies was 1 cm. For UV-vis and fluorescence titrations, stock solution of REDA-2PC (50 μM) was prepared in HEPES buffered (0.1 M; CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 9
H2O, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v; pH 7.4) solution. Working solutions of REDA-2PC and NCS− were prepared from their respective stock solutions. Fluorescence measurements were performed using 5 nm × 2.5 nm slit width. Except time dependent spectra, all the fluorescence and absorbance spectra were taken after 30 min of mixing of REDA-2PC with NCS−.
1 v/v; pH 7.4) solution. Working solutions of REDA-2PC and NCS− were prepared from their respective stock solutions. Fluorescence measurements were performed using 5 nm × 2.5 nm slit width. Except time dependent spectra, all the fluorescence and absorbance spectra were taken after 30 min of mixing of REDA-2PC with NCS−.
Effect of NCS− incubation on the REDA-2PC loaded HEK293 cells
HEK293 coated on fibronectin-coated cover slips18 were preloaded with REDA-2PC (10 μM, final concentration) for 15 min at 37 °C in the CaHT medium (132.0 mM NaCl, 4.2 mM KCl, 1.4 mM CaCl2, 1.0 mM MgCl2, 5.5 mM D-glucose, 10 mM HEPES/Tris, pH 7.4). The CaHT medium, being colorless, did not interfere with the fluorescence measurements and represents the normal extracellular milieu. The freshly prepared NCS− (100 mM) stock solution was diluted in CaHT up to the final concentration of 0.1 nM, 1 μM or 100 μM and co-incubated with probes for 15 min. After 15 min, cells were washed once with 1 mL CaHT, after which cover slips were placed in the incubation chamber containing CaHT (500 μL) and placed on the microscope.Effect of addition of NCS− to REDA-2PC and RTA loaded HEK293 cells
HEK293 cells on the fibronectin-coated cover slips were preloaded with REDA-2PC and RTA (10 μM, final concentration) for 15 min at 37 °C and then with CaHT in the absence (black lines) or presence (red lines) of NCS− (100 μM). After REDA-2PC or RTA loading, cells were placed in the incubation chamber containing CaHT (500 μL) with REDA-2PC or RTA (10 μM, final concentration), and measurement were started. At 60 s, the NCS− (500 μL) containing CaHT was added (100 μM, final concentration). At 200 s, CaHT (500 μL) was added as control only for REDA-2PC and again at 240 s, CaHT (500 μL) was added as control. Both the free and NCS− treated RTA lost their signals immediately when CaHT was added as control. The NCS− treated REDA-2PC hold its intracellular fluorescence up to 1500 μL of the added CaHT at the extracellular medium, while free REDA-2PC lost its signal after 1000 μL of the added CaHT as control. After measurements, average intracellular fluorescence of multiple cells was analyzed and corrected for background.Measurement of NCS− concentration in blood serum and milk
Fresh sheep blood was collected and allowed to clot without disturbance. The straw yellow color serum was collected and diluted 40 times in HEPES buffered (0.1 M; CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 9
H2O, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v; pH 7.4) solution. The solution was centrifuged at 2000 rpm for 5 min and the supernatant liquid was collected. Fluorescence intensities of the serum samples (1.0, 1.5 and 2.0 mL) were measured in the presence of REDA-2PC (50 μM) and after spiking NCS− (2 μM). The same procedure was applied for the 400 times diluted cow milk. The NCS− concentration of these samples was calculated using the linear regression of the fluorescence intensity of REDA-2PC (50 μM), observed up to 10 μM of the added NCS−, viz. intensity = 44.45 + 25.70 × [NCS−]. Results are listed in Tables S3 and S4.† The close proximity of the results obtained by the standard addition method clearly indicates the non-interference of complex components present in the blood serum and milk. The enzymes or proteins, which present in a human body fluid, may exhibit fluorescence due to the presence of amino acids (e.g. tryptophan, histidine, etc.). However, these amino acids have an excitation wavelength in the UV region. The excitation wavelength of REDA-2PC is in the visible region (λexc = 520 nm), and the interference of these enzymes or proteins were ruled out.
1 v/v; pH 7.4) solution. The solution was centrifuged at 2000 rpm for 5 min and the supernatant liquid was collected. Fluorescence intensities of the serum samples (1.0, 1.5 and 2.0 mL) were measured in the presence of REDA-2PC (50 μM) and after spiking NCS− (2 μM). The same procedure was applied for the 400 times diluted cow milk. The NCS− concentration of these samples was calculated using the linear regression of the fluorescence intensity of REDA-2PC (50 μM), observed up to 10 μM of the added NCS−, viz. intensity = 44.45 + 25.70 × [NCS−]. Results are listed in Tables S3 and S4.† The close proximity of the results obtained by the standard addition method clearly indicates the non-interference of complex components present in the blood serum and milk. The enzymes or proteins, which present in a human body fluid, may exhibit fluorescence due to the presence of amino acids (e.g. tryptophan, histidine, etc.). However, these amino acids have an excitation wavelength in the UV region. The excitation wavelength of REDA-2PC is in the visible region (λexc = 520 nm), and the interference of these enzymes or proteins were ruled out.
Density functional theory (DFT) based calculations
We have applied in the ADF/DFT ground state optimizations the BLYP-D3/TZP protocol. For the TD-DFT the ADF/B3LYP/TZP was applied including the COSMO15 solvent model. Deformation density contributions of the ETS-NOCV method were plotted based on the ADF-GUI interface.19Dynamics simulations
Molecular dynamics simulations at Car–Parrinello level was done by means of the CPMD software package, using a plane wave basis set with cutoff energy of 100 Ry, within a cubic cell of 16 Å in length. We have used the time step length of 4.134 atu (0.1 fs) and the inertia parameter for wavefunction dynamics (fictitious electron mass) is 500 amu. The simulation time was ∼11 ps. The temperature 298.15 K has been controlled via Nosé–Hoover chain thermostat. Valence electrons have been treated explicitly within the DFT formalism employing the PBE exchange–correlation functional Grimme's dispersion correction, whereas for epy inner electrons description the Goedecker type pseudopotentials have been used. VMD software package12e have been used for animation.ETS-NOCV bonding analysis
The Natural Orbitals for Chemical Valence (NOCV) have been derived from the Nalewajski–Mrozek valence theory as eigenvectors that diagonalize the deformation density matrix.13a,c It was shown that the natural orbitals for chemical valence pairs (ψ−k,ψk) decompose the differential density Δρ into NOCV-contributions (Δρk):where νk and M stand for the NOCV eigenvalues and the number of basis functions, respectively. Visual inspection of deformation density plots (Δρk) helps to attribute symmetry and the direction of the charge flow. In addition, these pictures are enriched by providing the energetic estimations, ΔEorb(k), for each Δρk within ETS-NOCV scheme.13b The exact formula, which links the ETS and NOCV methods, will be given in the next paragraph, after we briefly present the basic concept of ETS scheme. In this method the total bonding energy ΔEtotal between interacting fragments, exhibiting the geometry as in the combined complex, is divided into the three components: ΔEtotal = ΔEelstat + ΔEPauli + ΔEorb. The first term, ΔEelstat, corresponds to the classical electrostatic interaction between the promoted fragments as they are brought to their positions in the final complex. The second term, ΔEPauli, accounts for the repulsive Pauli interaction between occupied orbitals on the two fragments in the combined molecule. Finally, the last stabilizing term, ΔEorb represents the interactions between the occupied molecular orbitals of one fragment with the unoccupied molecular orbitals of the other fragment as well as mixing of occupied and virtual orbitals within the same fragment (inner-fragment polarization). This energy term may be linked to the electronic bonding effect coming from the formation of a chemical bond. In the combined ETS-NOCV scheme the orbital interaction term (ΔEorb) is expressed in terms of NOCV's eigenvalues (vk) as:
where FTSk,k are diagonal Kohn–Sham matrix elements defined over NOCV with respect to the transition state (TS) density (at the midpoint between density of the molecule and the sum of fragment densities). The above components ΔEorb(k) provide the energetic estimation of Δρk that may be related to the importance of a particular electron flow channel for the bonding between the considered molecular fragments. ETS-NOCV analysis13b was done based on the Amsterdam Density Functional (ADF) package in which this scheme was implemented.
Molecular electrostatic potential
The electrostatic potential V(r) of molecule at point “r”, due to nuclei and electrons, is given by:where ZA is the charge of nucleus at position RA and ρ(r) is the total electronic density. The sign of V(r) depends upon whether the positive contribution of the nuclei or negative from the electrons is dominant. Negative values of V(r) correspond to nucleophilic areas of molecule, whereas the positive to electrophilic regions.
Syntheses
Single crystal X-ray diffraction
The X-ray data for FEDA were collected at 150(2) K on a Mar345 image plate detector using Mo-Kα radiation (Xenocs Fox3D mirror). The data were integrated with the crysAlisPro software.20 The implemented empirical absorption correction was applied. The structures were solved by direct methods using the SHELXS-97 program21 and refined by full-matrix least squares on |F2| using SHELXL-97.21 Non-hydrogen atoms were anisotropically refined and the hydrogen atoms were placed on calculated positions in riding mode with temperature factors fixed at 1.2 times Ueq of the parent atoms. Figures were generated using the program Mercury.22 C22H18N2O4, Mr = 374.38 g mol−1, orthorhombic, space group Pbcn, a = 14.6159(5), b = 9.9444(3), c = 28.7738(12) Å, V = 4182.2(3) Å3, Z = 8, ρ = 1.189 g cm−3, μ(Mo-Kα) = 0.083 mm−1, reflections: 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 collected, 3809 unique, Rint = 0.123, R1(all) = 0.0505, wR2(all) = 0.1327.
115 collected, 3809 unique, Rint = 0.123, R1(all) = 0.0505, wR2(all) = 0.1327.
Acknowledgements
S. Mandal, A. Sahana and A. Banerjee are thankful to UGC and CSIR, New Delhi, for fellowships. Theoretical calculations were performed using PL-Grid Infrastructure and resources provided by ACC Cyfronet AGH.References
- (a) O. L. Oke, World Rev. Nutr. Diet., 1969, 11, 170 CrossRef CAS PubMed; (b) O. Agbai, J. Natl. Med. Assoc., 1986, 78, 1053 CAS.
- A. Cerami and J. M. Manning, Proc. Natl. Acad. Sci. U. S. A., 1971, 68, 1180 CrossRef CAS.
- (a) J. R. Riordan, J. M. Rommens, B.-S. Kerem, N. Alon, R. Rozhamel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J.-L. Chou, M. L. Drumm, M. C. Iannuzi, F. Collins and L.-C. Tsui, Science, 1989, 245, 1066 CAS; (b) B. Alkhouri, R. A. Denning, P. K. Chiaw, P. D. W. Eckford, W. Yu, C. Li, J. J. Bogojeski, C. E. Bear and R. D. Viirre, J. Med. Chem., 2011, 54, 8693 CrossRef CAS PubMed.
- A. Banerjee, A. Sahana, S. Lohar, I. Hauli, S. K. Mukhopadhyay, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia and D. Das, Chem. Commun., 2013, 49, 2527 RSC.
- S. Rastegarzadeh and Z. Moradpour, Anal. Lett., 2007, 40, 2993 CrossRef CAS.
- Z. Xu, K. H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601 CrossRef CAS PubMed.
- H. Chakrapani, A. E. Maciag, M. L. Citro, L. K. Keefer and J. E. Saavedra, Org. Lett., 2008, 10, 5155 CrossRef CAS PubMed.
- R. J. Ritchie and J. Gibson, J. Membr. Biol., 1987, 95, 131 CrossRef CAS.
- C. L. Andrew, A. R. Klemm and J. B. Lloyd, Biochim. Biophys. Acta, 1997, 1330, 71 CrossRef CAS.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- ADF2012.01: (a) G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931 CrossRef CAS and references therein; (b) E. J. Baerends, J. Autschbach, D. Bashford, A. Bérces, F. M. Bickelhaupt, C. Bo, P. M. Boerrigter, L. Cavallo, D. P. Chong, L. Deng, R. M. Dickson, D. E. Ellis, M. van Faassen, L. Fan, T. H. Fischer, C. Fonseca Guerra, A. Ghysels, A. Giammona, S. J. A. van Gisbergen, A. W. Götz, J. A. Groeneveld, O. V. Gritsenko, M. Grüning, F. E. Harris, P. van den Hoek, C. R. Jacob, H. Jacobsen,L. Jensen, G. van Kessel, F. Kootstra, M. V. Krykunov, E. van Lenthe, D. A. McCormack, A. Michalak, M. Mitoraj, J. Neugebauer, V. P. Nicu, L. Noodleman, V. P. Osinga, S. Patchkovskii, P. H. T. Philipsen, D. Post, C. C. Pye, W. Ravenek, J. I. Rodríguez, P. Ros, P. R. T. Schipper, G. Schreckenbach, M. Seth, J. G. Snijders, M. Solà, M. Swart, D. Swerhone, G. te Velde, P. Vernooijs, L. Versluis, L. Visscher, O. Visser, F. Wang, T. A. Wesolowski, E. M. van Wezenbeek, G. Wiesenekker, S. K. Wolff, T. K. Woo, A. L. Yakovlev and T. Ziegler, Theoretical Chemistry, Vrije Universiteit, Amsterdam Search PubMed.
- (a) CPMD, Copyright IBM Corp. 1990–2008, Copyright MPI für Festkörperforschung Stuttgart, 1997–2001; (b) R. W. Hockney, Methods Comput. Phys., 1970, 9, 136 Search PubMed; (c) J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed; (d) C. Hartwigsen, S. Goedecker and J. K. Hutter, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 3641 CrossRef CAS; (e) W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33 CrossRef CAS.
- (a) M. Mitoraj and A. Michalak, J. Mol. Model., 2007, 13, 347 CrossRef CAS PubMed; (b) A. Michalak, M. Mitoraj and T. Ziegler, J. Phys. Chem. A, 2008, 112, 1933 CrossRef CAS PubMed; (c) M. Mitoraj, A. Michalak and T. Ziegler, J. Chem. Theory Comput., 2009, 5, 962 CrossRef CAS PubMed.
- E. Runge and E. K. U. Gross, Phys. Rev. Lett., 1984, 52, 997 CrossRef CAS.
- A. Klamt, J. Phys. Chem., 1995, 99, 2224 CrossRef CAS.
- R. Kubin, J. Lumin., 1983, 27, 455 CrossRef CAS.
- E. Austin and M. Gouterman, Bioinorg. Chem., 1978, 9, 281 CrossRef CAS.
- T. de Groot, S. Verkaart, Q. Xi, R. J. Bindels and J. G. Hoenderop, J. Biol. Chem., 2010, 285, 28481 CrossRef CAS PubMed.
- O. Visser, P. Leyronnas, W. J. van Zeist and M. Lupki, ADF-GUI 2012.01, SCM, Amsterdam, The Netherlands, http://www.scm.com Search PubMed.
- Oxford Diffraction Data collection and data reduction, Version 171.34.40.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- I. J. Bruno, J. C. Cole, P. R. Edgington, M. Kessler, C. F. Macrae, P. McCabe, J. Pearson and R. Taylor, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 389 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Additional data, Fig. S1–S27 and Tables S1–S4. CCDC 959008. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra21838a |
| This journal is © The Royal Society of Chemistry 2015 |