Synthesis and diverse general oxidative cyclization catalysis of high-valent MoVIO2(HL) to ubiquitous heterocycles and their chiral analogues with high selectivity†
Nabyendu Pramanik,
Satinath Sarkar,
Dipanwita Roy,
Sudipto Debnath,
Sukla Ghosh,
Saikat Khamarui and
Dilip K. Maiti*
Department of Chemistry, University of Calcutta, 92 A. P. C. Road, Kolkata-700009, India. E-mail: dkmchem@caluniv.ac.in; Fax: +91-33-2351-9755; Tel: +91-33-2350-9937
First published on 17th November 2015
Abstract
The first synthesis and diverse oxidative cyclization catalysis properties of high-valent MoVI–triazole are demonstrated towards highly selective construction of benzimidazoles, benzothiazoles, isoxazolines, isoxazoles and their chiral analogues.
The discovery of a new metal complex and its diverse catalytic activity is a significant challenge in the chemical sciences. The transition metals of lower to medium oxidation states are now dominating the field of catalysis.1 Metal complexes of higher oxidation states have only a limited application in catalytic organic transformations. For example, metabolic oxidation, CO2-activated insertion, C–O hydrogenolysis and our recently reported oxidative cyclization are a few interesting organic transformations executed utilizing FeV–cytochrome P450, UV–carbamate, ZrIV–triflate and MnVI-nanoparticles, respectively.2 Inexpensive and readily available high-valent metals have tremendous potential in accommodating a number of ligand sites, functionalities and substrates, and bringing them in close proximity leading to the simultaneous construction of multiple homo- and heteroatomic bonds, and cyclic frameworks. The electronic and steric parameters of the high-valent metal simultaneously enable high selectivity, which is one of the key factors of an efficient catalyst. Additionally, the higher oxidation state of the catalyst provides a unique power for executing a simultaneous oxidation process in the presence of molecular oxygen or other stoichiometric oxidants. The higher oxidation state of biologically essential molybdenum compounds has attracted considerable attention, especially due to their outstanding enzymatic activities3 and olefin metathesis.4 Herein we communicate the discovery of high-valent MoVI–ONO complexes (Scheme 1), their properties, diverse general oxidative cyclization catalysis and selectivity for the most frequently synthesized ubiquitous heterocycles such as functionalized benzimidazoles, benzothiazoles, isoxazolines, isoxazoles and their chiral analogues.
Benzimidazoles are versatile compounds used in a wide range of scientific and industrial applications such as important building blocks for many organic syntheses, catalysis, fluorescence, chemosensing, crystal engineering, corrosion science, materials, organic electronics, medicinal research, pharmaceuticals, agrochemicals, textiles and cosmetics.5 The widely used oxidative cyclocondensation of o-phenylenediamines and aldehydes to benzimidazoles has a serious problem of forming two products6d namely 2-substituted and 1,2-disubstituted benzimidazoles (Table 1). In addition to the huge number of reports on the synthesis of valuable benzimidazoles,6 the development of an efficient, benign and highly selective strategy is still in demand, especially for affording 2-substituted benzimidazoles and their chiral analogues7 bearing free-NH for a wide range of applications in the nanoscience and pharmaceutical industry. The scope of the benign oxidative cyclization catalysis can be extended towards the synthesis of the therapeutically useful benzothiazoles utilising labile 2-aminothiophenols and aldehydes.8,9 Isoxazolines10 and isoxazoles11 are essential building blocks of numerous natural products, useful synthons, chiral macrocycles and bioactive compounds for a wide range of medicinal applications.12 In fact these are the most frequently synthesised five member heterocycles through 1,3-dipolar nitrile oxide cycloadditions.13,14
| Entry | Catalysta | Reaction conditionsb,c | Conversion (%) | Yieldd (%) |
|---|---|---|---|---|
| a Catalyst loading: 10 mol%.b Oxygen from air.c rt.d Isolated product after purification.e 8 mol%.f 7 mol%.g 10 mol%.h Not isolated. | ||||
| 1 | MoO2(HL) | DCM, MgSO4, 7 h | 98 | 68 |
| 2 | MoO2(HL) | PhMe, MgSO4, 24 h | 60 | 45 |
| 3 | MoO2(HL) | MeOH, MgSO4, 12 h | 40 | 25 |
| 4 | MoO2(HL) | MeCN, MgSO4, 16 h | 80 | 56 |
| 5 | MoO2(HL) | THF, MgSO4, 6 h | 100 | 86 |
| 6 | MoO2(HL)e | THF, MgSO4, 7 h | 100 | 86 |
| 7 | MoO2(HL)f | THF, MgSO4, 20 h | 90 | 79 |
| 8 | MoO2(HL), CeCl3g | THF, urea–H2O2, MgSO4, 6 h | 100 | 89 |
| 9 | MoO2(HL) | PhI(OAc)2, THF, MgSO4, 12 h | 100 | 55 |
| 10 | MoO2(HL) | THF, MgSO4, Ar, 9 h | 10 | 5h |
| 11 | MoO2(HL) | THF, MgSO4, O2, 6 h | 100 | 88 |
| 12 | MoO2(acac)2 | THF, MgSO4, 24 h | 50 | 35 |
The organometallic chemistry of 3,5-bis(2-hydroxyphenyl)-1H-1,2,4-triazoles (H3L, Scheme 1) was first studied by Ryabukhin in 1983.15 To the best of our knowledge, an MoVI-complex with an “ONO” ligand is unknown in the literature. We have devised a simple procedure to access MoVI–1,2,4-triazole complexes from MoO2(acac)2 under benign reaction conditions (Scheme 1). The structure of the new high-valent compound was established by single crystal X-ray crystallography (panel A, Fig. 1),16 NMR, FTIR, SQUID (panel B) and EPR (panel C) analyses. Interestingly the XRD structure of triclinic symmetry with a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group reveals a higher binding site of MoVI, which is associated with water and DMF [MoO2(HL)(H2O)DMF]. The loosely bound MoVI–N (HL), MoVI–O (H2O) and MoVI–O (DMF) may be useful for binding substrates during oxidative catalysis processes. The MoVI-complex is expected to be diamagnetic with a d0 electronic configuration,17 and the higher oxidation state species revealed an unexpectedly high magnetic moment, measured using SQUID (0.54 BM, panel B), with a hyperfine X-band EPR spectrum (panel C). The free-N–H of the complex may play a vital role during the catalytic process through transforming coordinated N–MoVI σ-bonds as per the requirement of the push–pull mechanism of the catalytic cycles.
space group reveals a higher binding site of MoVI, which is associated with water and DMF [MoO2(HL)(H2O)DMF]. The loosely bound MoVI–N (HL), MoVI–O (H2O) and MoVI–O (DMF) may be useful for binding substrates during oxidative catalysis processes. The MoVI-complex is expected to be diamagnetic with a d0 electronic configuration,17 and the higher oxidation state species revealed an unexpectedly high magnetic moment, measured using SQUID (0.54 BM, panel B), with a hyperfine X-band EPR spectrum (panel C). The free-N–H of the complex may play a vital role during the catalytic process through transforming coordinated N–MoVI σ-bonds as per the requirement of the push–pull mechanism of the catalytic cycles.
To explore the oxidative catalytic activity of the new high-valent metal complex we initially executed a cyclocondensation cum oxidation reaction using 10 mol% of MoO2(HL)(H2O)(DMF), o-phenylenediamine (1a, 1 mmol), benzaldehyde (2a, 1.1 mmol), desiccant MgSO4 and solvent dichloromethane under aerobic conditions at ambient temperature (entry 1, Table 1). Gratifyingly the desired product 2-(phenyl)-1H-benzimidazole (3a, Fig. 1) was obtained with excellent selectivity. The possible byproduct 4a (Scheme 2) was not found in the post reaction mixture, which is the major concern of most of the reported methods. The moderate yield (68%) of the desired product 3a (entry 1, Table 1) led us to optimise the reaction using nonpolar, polar and protic solvents (entries 2–5), and the yield of the benzimidazole 3a was significantly improved (86%, entry 5) in THF medium, probably due to better stabilization of the intermediates in the transition state through coordination of THF as ligand. However we found a small amount of corresponding aldodimine, which was not converted into 3a. The catalyst loading was optimised to 8 mol% (entries 6–8). There was a little improvement in the yield using co-catalyst CeCl3 (10 mol%)9d,18 and urea–H2O2, but not with PhI(OAc)2 (ref. 19) (entries 8 and 9). The role of molecular oxygen as a stoichiometric oxidant in the oxidative cyclocondensation process (entries 10 and 11) was essential and it was verified by conducting two separate experiments, in the absence (entry 10) and presence of oxygen (entry 11). Our attempt to use simple MoO2(acac)2 as a catalyst (entry 12) was unsuccessful, which indicates that the presence of HL around the MoVI is essential for empowering it as a work-horse in the catalysis.
With this promising result in hand (entry 6, Table 1), the versatility of the benign synthetic approach involving the catalyst MoO2(HL)(H2O)DMF was successfully examined (Scheme 2) by the cyclocondensation cum oxidation of various functionalised aromatic aldehydes (2b–f) with different o-phenylenediamines (1a–c) at ambient temperature under similar reaction conditions to afford the corresponding benzimidazoles (3b–m) with a fast reaction rate (6–7 h) and high yield (74–86%). Electron-deficient, electron-rich and heteroaromatic substituents were tolerated in this catalytic process. Our aim was to develop a benign strategy for synthesising sensitive and labile molecules. This benign strategy was utilized successfully towards the direct synthesis of valuable glyceral- and glycal-based chiral benzimidazoles (3n–p).
Next, we explored the possibility of synthesising another ubiquitous framework, benzothiazole, using the powerful high-valent catalyst (Scheme 3). Gratifyingly the optimised reaction conditions (entry 6, Table 1) afforded the desired product 2-phenylbenzothiazole (6a, Scheme 3) from 2-aminothiophenol (5a) and benzaldehyde (2a). However, the yield (45%) and reaction time (24 h) were not encouraging due to oxidative dimerization of precursor 2-aminothiophenol (5a) through the formation of S–S bonds in the presence of molecular oxygen. After several attempts using various potential oxidants [PhI(OAc)2, PhIO, NMPO etc.], CeCl3 (10 mol%) was found to be an efficient co-catalyst for the oxidative cyclization process using non-aqueous urea–hydrogen peroxide as a stoichiometric oxidant. Herein the role of CeCl3 (ref. 20) is to supply oxygen from urea–hydrogen peroxide for the oxidative cyclization process. The heterocycle was obtained in high yield (83%) after 4 h at room temperature under an argon atmosphere. We have executed the reaction in the absence of the MoO2(HL)(H2O)DMF catalyst and the reaction was unsuccessful. Further, the dehydrative cyclisation cum oxidation process was carried out between 5a bearing an oxidation prone –SH group, and functionalised aromatic aldehydes (2b–i), which rapidly (4–5 h) furnished the respective benzothiazoles (6b–g) in excellent yields (82–89%). The sugar-based chiral benzothiazole 6h was successfully synthesised with high yield (82%). Most of the reported catalytic methods are incapable of direct access to the sugar-based chiral benzothiazole. The in situ generated urea was recovered and recycled through the formation of urea–hydrogen peroxide using hydrogen peroxide.
After investigating the applicability of the new high-valent MoO2(HL)(H2O)DMF catalyst, it was further examined for the synthesis of another frequently synthesised five member N-heterocycle isoxazoline generated via intermolecular 1,3-dipolar cycloaddition of nitrile oxides and functionalised olefins. The benzaldehyde aldoxime (7a) and ethyl acrylate (8a) were treated with the combo catalyst MoO2(HL)(H2O)DMF (8 mol%)–CeCl3 (10 mol%) at ambient temperature. To our delight the desired isoxazoline 9a (Scheme 4) was generated selectively without the formation of the corresponding other regioisomer 10a. However, the cycloaddition reaction was arrested in the absence of the MoO2(HL)(H2O)DMF catalyst. The versatility of the benign synthetic approach is verified with several precursors bearing electron-rich and electron-deficient aromatic substituents, which were tolerated in this benign approach to achieve isoxazolines 9b–l with excellent regioselectivity. The respective regioisomer (10) was not found using this powerful catalysis process, which is the major concern of the existing methods. The heterocycle based isoxazoline (9i) and sugar-based chiral isoxazolines (9l–o) were also achieved in good yields.
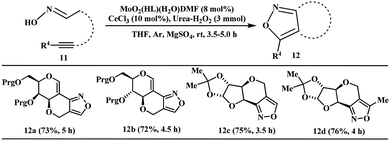
The versatility of this benign catalysis of high-valent MoVIO2(HL)(H2O)DMF was also verified in the asymmetric intramolecular cycloaddition reaction of nitrile oxide with alkynes for the synthesis of chiral fused-isoxazoles of allyl-substituted glycalaldoximes and pentose sugar analogues (11, Scheme 5). This synthetic tool with sugar-based nitrile oxides provides an excellent opportunity for constructing nature-like and unnatural organic molecules. Under the catalytic conditions, the chiral oximes (11a–d) underwent smoothly the reaction in a highly stereoselective fashion to produce sugar-based chiral isoxazoles (12a–d) in good yields (72–76%). The CeCl3 co-catalyst has the crucial role as an oxygen carrier from urea–H2O2 to the MoVI-catalyst for executing the oxidative intramolecular dipolar cycloaddition reactions.
Possible mechanisms of the diverse cyclocondensation cum oxidation and oxidative cycloaddition reactions are shown in Scheme 6. The first step for the oxidative cyclocondensation cum oxidation is expected to proceed through coordination of the high-valent catalyst with the substrates (I, cycle A, Scheme 6) and subsequent selective cyclisation to form intermediate II. It smoothly releases the desired products, benzimidazoles (3) and benzothiazoles (5), through reductive elimination of the MoV-complex (III), which is eventually regenerated to the MoVI-complex for the next catalytic cycle through oxidation with oxygen or CeCl3–urea·H2O2. The eliminating C–H of intermediate II for benzimidazole is relatively more acidic than that of benzothiazole due to the presence of two more electron withdrawing C–N bonds. Thus the molecular oxygen–MoVI-complex easily transforms II into benzimidazole (3). The highly regioselective synthesis of isoxazolines (9) and isoxazoles (12) also followed a similar catalytic cycle (cycle B, Scheme 6). Herein the role of CeCl3 is as an oxygen carrier to MoV–OH for regenerating the active MoVI-catalyst using H2O2–urea as a stoichiometric oxidant.20 However involvement of co-catalyst CeCl3 (IV → V) in the cyclisation step can’t be avoided. The high regioselectivity in all of the oxidative cyclisation processes can be explained in terms of the strong binding of the substrates and successive hetero- and homonuclear coupling through the large binding site of the powerful high-valent metal-complex during the formation of I → II (A) and IV → V (B).
In conclusion, we have discovered a high-valent MoO2(HL)(H2O)(DMF) complex with the rarely used H3L ligand and found it to be a powerful catalyst for oxidative cyclocondensation using oxygen as a stoichiometric oxidant to furnish 2-substituted benzimidazoles selectively. In contrast to the common epoxidation catalysis by MoVI-complexes, herein the MoO2(HL)(H2O)(DMF) complex has shown the diverse highly selective synthesis of ubiquitous 2-substituted benzimidazoles, 2-substituted benzothiazoles, isoxazolines, isoxazoles and their chiral analogues. Herein we demonstrated the MoVI(HL) as an attractive catalyst for developing benign, simple and general oxidative cyclization processes with excellent selectivity. We anticipate that the discovery of a new high-valent catalyst, its novel properties and robust oxidative cyclization catalysis will find considerable application in chemical science towards devising new strategies for functionalising molecules and discovering prospective new high-valent catalysts.
Acknowledgements
Financial support from DST (SR/S1/OC-05/2012 and SR/S5/GC-04/2012) and research fellowships from UGC (D. S. Kothari and SRF) and CSIR (JRF and SRF) are gratefully acknowledged.Notes and references
- (a) G. Rothenberg, Catalysis: concepts and green applications, Wiley-VCH Verlag GmbH and Co. KGaA, Weinheim, 2008 Search PubMed; (b) K. A. Jørgensen, Angew. Chem., Int. Ed., 2000, 39, 3558 CrossRef; (c) M. Peplow, Nature, 2013, 495, S10–S11 CrossRef CAS PubMed; (d) S. Ghosh, S. Khamarui, K. S. Gayen and D. K. Maiti, Sci. Rep., 2013, 3, 2987 Search PubMed.
- (a) L. Que Jr and W. B. Tolman, Nature, 2008, 455, 333 CrossRef PubMed; (b) S. C. Bart, C. Anthon, F. W. Heinemann, E. Bill, N. M. Edelstein and K. Meyer, J. Am. Chem. Soc., 2008, 130, 12536 CrossRef CAS PubMed; (c) Z. Li, R. S. Assary, A. C. Atesin, L. A. Curtiss and T. J. Marks, J. Am. Chem. Soc., 2014, 136, 104 CrossRef CAS PubMed; (d) S. Khamarui, Y. Saima, R. M. Laha, S. Ghosh and D. K. Maiti, Sci. Rep., 2015, 5, 8636 CrossRef CAS PubMed.
- (a) C. J. Doonan, D. J. Nielsen, P. D. Smith, J. M. White, G. N. George and C. G. Young, J. Am. Chem. Soc., 2006, 128, 305 CrossRef CAS PubMed; (b) L. M. R. Hill, M. K. Taylor, V. Wee, L. Ng and C. G. Young, Inorg. Chem., 2008, 47, 1044 CrossRef CAS PubMed; (c) F. Biaso, B. Burlat and B. Guigliarelli, Inorg. Chem., 2012, 51, 3409 CrossRef CAS PubMed.
- (a) K. A. Jørgensen, Chem. Rev., 1989, 89, 431 CrossRef; (b) R. R. Schrockand and A. H. Hoveyda, Angew. Chem., Int. Ed., 2003, 42, 4592 CrossRef PubMed; (c) F. E. Kühn, A. M. Santos and M. Abrantes, Chem. Rev., 2006, 106, 2455 CrossRef PubMed; (d) R. Sanz and M. R. Pedrosa, Curr. Org. Synth., 2009, 6, 239 CrossRef CAS; (e) L. Ondi, J. Varga, A. Bucsai, F. Toth, K. Lorincz, C. Hegedus, E. Robbe and G. Frater, PCT Int. Appl., WO 2014139679 A2 20140918, 2014.
- (a) W. B. Pratt, Chemotherapy of Infection, Oxford University Press, New York, 1997 Search PubMed; (b) A. A. Spasov, I. N. Yozhitsa, L. I. Bugaeva and V. A. Anisimova, J. Pharm. Chem., 1999, 33, 232 CrossRef CAS; (c) N. Singh and D. O. Jang, Org. Lett., 2007, 9, 1991 CrossRef CAS PubMed; (d) J. M. Roque, T. Pandiyan, J. Cruz and E. García-Ochoa, Corros. Sci., 2008, 50, 614 CrossRef CAS; (e) V. D. Umare, V. N. Ingle and R. K. Wanare, Int. J. ChemTech Res., 2009, 1, 314 CAS; (f) Y. Bansal and O. Silakari, Bioorg. Med. Chem., 2012, 20, 6208 CrossRef CAS PubMed; (g) S. Samai and K. Biradha, Chem. Mater., 2012, 24, 1165 CrossRef CAS; (h) S. Asthana, S. Shukla, A. V. Vargiu, M. Ceccarelli, P. Ruggerone, G. Paglietti, M. E. Marongiu, S. Blois, G. Giliberti and P. L. Colla, Biochemistry, 2013, 52, 3752 CrossRef CAS PubMed; (i) U.-H. Jin, S.-B. Kim and S. Safe, Chem. Res. Toxicol., 2015, 28, 907 CrossRef CAS PubMed.
- (a) P. N. Preston, Benzimidazoles In The Chemistry of Heterocyclic Compounds, ed. A. Weissberger and E. C. Taylor, John Wiley and Sons, New York, 1981, p. 40 Search PubMed; (b) X. Deng, H. McAllister and N. S. Mani, J. Org. Chem., 2009, 74, 5742 CrossRef CAS PubMed; (c) Y. Kim, M. R. Kumar, N. Park, Y. Heo and S. Lee, J. Org. Chem., 2011, 76, 9577 CrossRef CAS PubMed; (d) R. Chebolu, D. N. Kommi, D. Kumar, N. Bollineni and A. K. Chakraborti, J. Org. Chem., 2012, 77, 10158 CrossRef CAS PubMed; (e) S. S. Panda, R. Malik and S. C. Jain, Curr. Org. Chem., 2012, 16, 1905 CrossRef CAS; (f) J. Li, S. Bénard, L. Neuville and J. Zhu, Org. Lett., 2012, 14, 5980 CrossRef CAS PubMed; (g) G. Bai, X. Lan, X. Liu, C. Liu, L. Shi, Q. Chen and G. Chen, Green Chem., 2014, 16, 3160 RSC; (h) S. Dhole, M. Selvaraju, B. Maiti, K. Chanda and C.-M. Sun, ACS Comb. Sci., 2015, 17, 310 CrossRef CAS PubMed; (i) M. Gurry, M. Sweeney, P. McArdle and F. Aldabbagh, Org. Lett., 2015, 17, 2856 CrossRef CAS PubMed.
- (a) F. M. Rivas, A. J. Giessert and S. T. Diver, J. Org. Chem., 2002, 67, 1708 CrossRef CAS PubMed; (b) P. B. Hitchcock, A. C. C. Hodgson and G. J. Rowlands, Synlett, 2006, 2625 CAS; (c) M. Vojtech, M. Petrušová, E. Sláviková, S. Bekešová and L. Petrus, Carbohydr. Res., 2007, 342, 119 CrossRef CAS PubMed; (d) D. K. Maiti, S. Halder, P. Pandit, N. Chatterjee, D. de Joarder, N. Pramanik, Y. Saima, A. Patra and P. K. Maiti, J. Org. Chem., 2009, 74, 8086 CrossRef CAS PubMed; (e) N. A. Mirgane and A. V. Karnik, Chirality, 2011, 23, 404 CrossRef CAS PubMed; (f) H. Xu, H. Tian, L. Zheng, Q. Liu, L. Wang and S. Zhang, J. Heterocycl. Chem., 2012, 49, 1108 CrossRef CASS. Joardar, A. Bhattacharyya and S. Das, Synthesis, 2014, 46, 3121 CrossRef CAS; (g) C. Najera and M. Yus, Tetrahedron Lett., 2015, 56, 2623 CrossRef CAS.
- (a) K.-F. Inamato, C. Hasegawa, K. Hiroya and T. Doi, Org. Lett., 2008, 10, 5147 CrossRef PubMed; (b) H. Wang, L. Wang, J. Shang, X. Li, H. Wang, J. Guia and A. Lei, Chem. Commun., 2012, 48, 76 RSC; (c) C. Yu, K. Lee, Y. You and E. J. Cho, Adv. Synth. Catal., 2013, 355, 1471 CrossRef CAS; (d) Y. Sun, H. Jiang, W. Wu, W. Zeng and X. Wu, Org. Lett., 2013, 15, 1598 CrossRef CAS PubMed; (e) X. Zhang, W. Zeng, Y. Yang, H. Huang and Y. Liang, Org. Lett., 2014, 16, 876 CrossRef CAS PubMed; (f) N. P. Prajapati, R. H. Vekariya, M. A. Borad and H. D. Patel, RSC Adv., 2014, 4, 60176 RSC; (g) T. Guntreddi, R. Vanjari and K. N. Singh, Org. Lett., 2015, 17, 976 CrossRef CAS PubMed.
- (a) M. J. Costanzo, H. R. Almond Jr, L. R. Hecker, M. R. Schott, S. C. Yabut, H.-C. Zhang, P. Andrade-Gordon, T. W. Corcoran, E. C. Giardino, J. A. Kauffman, J. M. Lewis, L. de Garavilla, B. J. Haertlein and B. E. Maryanoff, J. Med. Chem., 2005, 48, 1984 CrossRef CAS PubMed; (b) S. Kini, S. P. Swain and A. M. Gandhi, Indian J. Pharm. Sci., 2007, 69, 46 CrossRef CAS; (c) T. C. R. Miller, T. J. Rutherford, K. Birchall, J. Chugh, M. Fiedler and M. Bienz, ACS Chem. Biol., 2014, 9, 2864 CrossRef CAS PubMed; (d) R. S. Keri, M. R. Patil, S. A. Patil and S. Budagumpi, Eur. J. Med. Chem., 2015, 89, 207 CrossRef CAS PubMed.
- (a) A. P. Kozikowski, Acc. Chem. Res., 1984, 17, 410 CrossRef CAS; (b) A. A. Fuller, B. Chen, A. R. Minter and A. K. Mapp, J. Am. Chem. Soc., 2005, 127, 5376 CrossRef CAS PubMed; (c) J. Sengupta, R. Mukhopadhyay, A. Bhattacharjya, M. M. Bhadbhade and G. V. Bhosekar, J. Org. Chem., 2005, 70, 8579 CrossRef CAS PubMed; (d) S. Tang, J. He, Y. Sun, L. He and X. She, J. Org. Chem., 2010, 75, 1961 CrossRef CAS PubMed; (e) L. Zhu, G. Wang, Q. Guo, Z. Xu, D. Zhang and R. Wang, Org. Lett., 2014, 16, 5390 CrossRef CAS PubMed.
- (a) V. Singh, G. P. Yadav, P. R. Maulik and S. Batra, Synthesis, 2006, 12, 1995 Search PubMed; (b) K. C. Coffman, T. A. Palazzo, T. P. Hartley, J. C. Fettinger, D. J. Tantillo and M. J. Kurth, Org. Lett., 2013, 15, 2062 CrossRef CAS PubMed.
- (a) M. J. Choi, E. S. No, D. A. Thorat, J. W. Jang, H. Yang, J. Lee, H. Choo, S. J. Kim, C. S. Lee, S. Y. Ko, J. Lee, G. S. Nam and A. N. Pae, J. Med. Chem., 2013, 56, 9008 CrossRef CAS PubMed; (b) R. Kakarla, J. Liu, D. Naduthambi, W. Chang, R. T. Mosley, D. Bao, H. M. M. Steuer, M. Keilman, S. Bansal, A. M. Lam, W. Seibel, S. Neilson, P. A. Furman and M. J. Sofia, J. Med. Chem., 2014, 57, 2136 CrossRef CAS PubMed; (c) C. Zhaoand and J. E. Casida, J. Agric. Food Chem., 2014, 62, 1019 CrossRef PubMed; (d) J. E. Casida, Chem. Res. Toxicol., 2015, 28, 560 CrossRef CAS PubMed.
- (a) J. W. Bode and E. M. Carreira, J. Am. Chem. Soc., 2001, 123, 3611 CrossRef CAS PubMed; (b) E. Y. Schmidt, I. V. Tatarinova, E. V. Ivanova, N. V. Zorina, I. A. Ushakov and B. A. Trofimov, Org. Lett., 2013, 15, 104 CrossRef CAS PubMed; (c) A. Yoshimura, K. R. Middleton, A. D. Todora, B. J. Kastern, S. R. Koski, A. V. Maskaev and V. V. Zhdankin, Org. Lett., 2013, 15, 4010 CrossRef CAS PubMed.
- (a) K. V. Gothelf and K. A. Jørgensen, Chem. Rev., 1998, 98, 863 CrossRef CAS PubMed; (b) H. Pellissier, Tetrahedron, 2007, 63, 3235 CrossRef CAS; (c) V. Nair and T. D. Suja, Tetrahedron, 2007, 63, 12247 CrossRef CAS; (d) A. Sperança, B. Godoi and G. Zeni, J. Org. Chem., 2013, 78, 1630 CrossRef PubMed; (e) E. B. Castillo-Contreras, A. M. Stahl and G. R. Dake, J. Org. Chem., 2014, 79, 7250 CrossRef CAS PubMed; (f) R. Harigae, K. Moriyama and H. Togo, J. Org. Chem., 2014, 79, 2049 CrossRef CAS PubMed; (g) P. Gao, H.-X. Li, X.-H. Hao, D.-P. Jin, D.-Q. Chen, X.-B. Yan, X.-X. Wu, X.-R. Song, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2014, 16, 6298 CrossRef CAS PubMed.
- (a) Y. I. Ryabukhin, L. N. Faleeva and V. G. Korobkova, Chem. Heterocycl. Compd., 1983, 19, 332 CrossRef; (b) J. G. Haasnoot, Coord. Chem. Rev., 2000, 200, 131 CrossRef; (c) M. H. Klingele and S. Brooker, Coord. Chem. Rev., 2003, 241, 131 CrossRef.
- CCDC no of MoO2(HL)(H2O)DMF: 1400488.†.
- E. I. Stiefel, Prog. Inorg. Chem., 1977, 22, 1 CrossRef CAS.
- (a) G. Bartoli, E. Marcantoni, M. Marcolini and L. Sambri, Chem. Rev., 2010, 110, 6104 CrossRef CAS PubMed; (b) R. Properzi and E. Marcantoni, Chem. Soc. Rev., 2014, 43, 779 RSC.
- (a) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (b) S. Khamarui, D. Sarkar, P. Pandit and D. K. Maiti, Chem. Commun., 2011, 47, 12667 RSC; (c) A. Parra and S. Reboredo, Chem.–Eur. J., 2013, 19, 17244 CrossRef CAS PubMed; (d) S. Khamurai, R. Maiti and D. K. Maiti, Chem. Commun., 2015, 51, 384 RSC.
- D. K. Maiti, S. Halder, P. Pandit, N. Chatterjee, D. D. Joarder, N. Pramanik, Y. Saima, A. Patra and P. K. Maiti, J. Org. Chem., 2009, 74, 8086 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and NMR spectra. CCDC 1400488. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra21825j |
| This journal is © The Royal Society of Chemistry 2015 |