Gold nanoparticles with high densities of small protuberances on nanocluster cores with strong NIR extinction†
Ameya U. Borwankara,
Brian W. Willseya,
April Twua,
Jessica J. Hunga,
Robert J. Stovera,
Tianyi W. Wangb,
Marc D. Feldmancd,
Thomas E. Milnerb,
Thomas M. Trusketta and
Keith P. Johnston*a
aMcKetta Department of Chemical Engineering, The University of Texas at Austin, USA. E-mail: kpj@che.utexas.edu
bDepartment of Biomedical Engineering, The University of Texas at Austin, USA
cSouth Texas Veterans Health Care System, San Antonio, TX 78229, USA
dDivision of Cardiology, Department of Medicine, University of Texas Health Science Center at San Antonio, San Antonio, TX 78229, USA
First published on 25th November 2015
Abstract
Plasmonic nanoparticles with sizes well below 100 nm and high near infrared (NIR) extinction are of great interest in biomedical imaging. Herein we present ∼60 nm Au nanoparticles with high NIR absorbance at wavelengths ranging from 700 nm to 1100 nm, which were synthesized under kinetic control. A high surface density of protuberances is grown on ∼30 nm nanocluster cores, which are composed of ∼10 nm primary particles. The high NIR extinction is produced by a combination of the close proximity of the primary particles in the cores, the high surface density of protuberances, and the high aspect ratio of the length of the protuberances to the diameter of the primary particles. When the Au precursor was reduced more slowly at a higher pH of 9.3, the growth was thermodynamically controlled and the nanocluster cores relaxed to spheres. This concept of self-assembly during reaction to change the morphology of nanoclusters and decorated nanoclusters, may be expected to be applicable to a wide variety of systems by balancing kinetic and thermodynamic control, along with the colloidal interactions.
Introduction
Gold nanoparticles with controlled optical and surface properties are of great interest in biomedical imaging and therapy,1,2 catalysis,3 energy,4,5 and plasmonic sensing.6,7 For biomedical imaging and therapy, the surface plasmon resonance (SPR) of Au nanocrystals may be tuned to the near infrared (NIR) region (800–1100 nm) where water, soft tissues, and blood absorb minimally.8 Furthermore, nanoparticles smaller than ∼60 nm are desirable for appropriate blood residence times and cellular uptake into targeted sites including cancerous tumors and atherosclerotic plaques.9–14 For Au nanospheres in water, the SPR peak is at 520 nm.15 For non-spherical morphologies, hybridization between dipoles and higher-order multipoles produces a red shift in the SPR to the NIR region for various shapes of monolithic particles including Au nanorods,16–20 nanoshells,1,21–23 nanocages,24 and nanostars (and similar morphologies like nanoflowers or nanourchins).25–42 For the simpler geometry of a single tip on a spherical core, the hybridization of the individual SPRs is well understood theoretically.43 In the case of nanostars it has been demonstrated experimentally that both the size and number of tips on the cores influence the SPR. The extent of the red shift of the hybridized SPR into the NIR region increases with aspect ratio of tip length to sphere diameter and the number of tips per spherical core.25,43,44 In addition to studies of monolithic particles, recent efforts are underway to determine shifts in the SPR for self-assembled nanoparticle clusters of closely-spaced primary nanoparticles with either random45–47 or controlled48–51 spacing between the particles. Insight into the effects of multi-body interactions on the SPR of nanoclusters may be gained from model systems composed of a pair of closely spaced particles as a function of the interparticle spacing.51,52Also, design rules are being developed to control the shape of monolithic Au nanoparticles by balancing thermodynamically controlled and kinetically controlled growth of Au on seeds.24,53–55 In the reaction-controlled regime, the morphologies consist of higher surface energy facets compared to the facets seen for the thermodynamically favored regime, which is favored by slower reaction rates.56 Based on the same principle, nanostar-like particles have been formed by directed growth on facets of Au seeds, with polymers or surfactants used to block certain selected facets.25–36 The length of the tips or arms of the stars may be controlled by varying the stoichiometric ratio of the Au3+ precursor to the core particles and also the precursor reduction rate.25
As an alternative to monolithic particles, nanoparticle clusters have been formed by colloidal assembly of primary particles in aqueous media for Au coated iron oxide,2,57 Au spheres,45–47 and CdSe quantum dots,58 and in organic media for TiO2 nanospheres (3 nm) or rods (3 nm to 6 nm).59 In some cases, the cluster size may be controlled by balancing long-ranged repulsion and short-ranged attraction. For example spherical Au particles were self-assembled into reversible (biodegradable) nanoclusters with sizes ranging from 30 to 100 nm by tuning colloidal interactions to achieve strong NIR extinction even at 1024 nm.45–47 Chains of Au particles were also formed by linking particles together using a polymerase chain reaction resulting in a shift of the absorbance to the NIR region.50 In a novel approach, Au spheres termed as satellites were also attached to a nanostar to increase the NIR extinction in the 800–1000 nm range.60 Recently, clusters formed from ∼100 nm long Au nanorods61 or ∼100 nm long nanodumbells62 have also been shown to shift the SPR peak into the NIR region out to 1500 nm.
Despite advances in controlling the shape of monolithic nanocrystals and self-assembled nanoclusters, it remains highly challenging to design nanoparticles with high extinction coefficients for wavelengths ranging from 800–1100 nm, particularly for particles smaller than 60 nm.29,34,37,39,63–66 For Au nanostars, long tips (and thus large particles) are required to get a sufficient red shift given the relatively large cores (∼10 to 30 nm).25,40,41,67–69 Indeed, only a few studies report particles on the order of 50 nm with strong NIR absorbance from 700–800 nm, namely, nanohexapods,56 nanocages24 and nanoclusters.2,47 In nearly all previous studies, the absorbance drops significantly at wavelengths greater than 1000 nm. In order to produce a high extinction at wavelengths greater than 1000 nm, the particle size is often >100 nm typically about 150 nm.70 Consequently novel concepts are needed to synthesize nanoparticles with a sufficient degree of asymmetry for broad NIR absorbance in small particles (<60 nm), as favored for biomedical applications.
Herein, we synthesize ∼60 nm Au nanoparticle clusters (hereafter termed as nanoclusters for brevity) exhibiting intense and broad NIR extinction from 700 to 1100 nm by reduction of HAuCl4 with hydroxylamine in the presence of either carboxymethyl dextran (CMD) or dextran. Reactions were performed at pH 8.7 and below to attempt to achieve kinetic control of the reaction and avoid relaxation of the growing particles to the thermodynamically favored state of spheres. The objective was to design small nanocluster cores formed by self-assembly of closely-spaced small primary particles and to then further decorate the cores with a large number of small protuberances as shown in Scheme 1. The change in the reaction pH enables different particle properties as a result of changed particle shapes. The large number of reactive sites on the ensemble of high surface area primary particles within the nanoclusters offers the opportunity to form a high density of protuberances per nanocluster. The nanoparticles exhibit greater extinction at 1000 nm than observed for similar sized particles with tips grown on monolithic cores as a consequence of the higher ratio of protuberance length to primary particle diameter. The low coverage of the weakly interacting polymer on Au is needed so that the primary particles are close enough together to produce strong NIR extinction through hybridization. At higher pH, slower precursor reduction forms spherical particles.
 | ||
| Scheme 1 Thermodynamically controlled growth at high pH versus kinetically controlled growth at lower pH values. | ||
At high pH (9.3), the clusters fill in or relax to form regular spherical shaped particles while at low pH (8.7), the clusters have further faceted growth of protuberances on them. At even lower pH (7.5), there is further faceted growth leading to star shaped particles.
The hybridization of the SPR from the large number of ∼10 nm long protuberances on similarly sized ∼10 nm primary particles (high aspect ratio of protuberance length/primary particle diameter) is shown to contribute to a large red-shift, as anticipated from previous simulation studies with a tip on a spherical core.25,43 The close spacings of Au primary particles in the nanocluster core further contribute to the strong NIR extinction from 700 to 1100 nm, despite the small size of the Au nanoclusters. Additionally, the polydispersity in the shape and size of these nanoparticles results in a broader spectra, with high extinction all the way to 1100 nm with an absorbance peak at 700 nm.
Materials and methods
Materials
All reagents used were analytical grade. Ammonium hydroxide, hydroxylamine hydrochloride, and anhydrous dextrose were purchased from Fisher Chemicals (Fairlawn, NJ). Carboxymethyl dextran sodium salt (MW = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and dextran (MW = 10
000) and dextran (MW = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) from Sigma Aldrich (St. Louis, MO), and tetrachloroauric acid trihydrate from MP Biomedicals LLC (Solon, Ohio). Fifty and 100 nm diameter gold nanourchins were purchased from Cytodiagnostics (Ontario, Canada).
000) from Sigma Aldrich (St. Louis, MO), and tetrachloroauric acid trihydrate from MP Biomedicals LLC (Solon, Ohio). Fifty and 100 nm diameter gold nanourchins were purchased from Cytodiagnostics (Ontario, Canada).
Synthesis of Au clusters – first iteration
Au nanocluster synthesis followed a modified procedure utilized previously for making Au/iron oxide composite nanoclusters.2 For carboxymethyl dextran coated nanoclusters, a 70.4 mL aqueous reducing solution was prepared at room temperature with 2.0 mM NH2OH·HCl, 0.3 mM carboxymethyl dextran, and 475 mM dextrose (mild reducing agent). Synthesis of dextran nanoclusters followed the same procedure with the same concentration (0.3 mM) of dextran. The pH of the solution was adjusted to pH 7.5, 8.7 or 9.3 using a solution of 7% ammonium hydroxide in water and was observed to decrease by about 0.1 units after addition of the precursor. Under rigorous stirring, a given volume of aqueous Au3+ precursor at a concentration of 12.7 mM was rapidly injected into the reducing solution to achieve the desired Au3+ precursor concentration. Reactions began to exhibit an initial blue color at ∼2 min which transitioned to pink for pH 9.3 and remained blue for lower pH reactions. The reaction mixture was allowed to sit for about 10 minutes and the particles were recovered or additional iterations of Au were added. After each iteration, including the first one, ∼10 minutes were allowed for reaction. The color was always seen to become constant within the ten minutes. For each iteration, a given amount of Au3+ precursor solution (0.1 mL) at 12.7 mM Au3+ precursor was injected in order to achieve the desired concentration of Au3+ precursor in solution. Final samples were concentrated by centrifugation at 6000 rpm for 7 min, after which the supernatant was decanted. The clusters were re-dispersed into about 0.5 mL of DI water with ∼1 min bath sonication to help remove the particles stuck to the walls of the centrifuge tube. Finally, this concentrated dispersion was diluted in DI H2O to various levels depending upon the characterization procedure.Quenching of reactions for temporal experiments
For stopping the reaction at intermediate stages to observe the temporal evolution of the Au nanoparticles, mercaptoacetic acid was used to complex with the unreacted Au precursor. A stoichiometric excess of about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of the mercaptoacetic acid was added to the reaction mixture at the desired timepoints and upon this addition, the color transition was observed to stop immediately.
1 of the mercaptoacetic acid was added to the reaction mixture at the desired timepoints and upon this addition, the color transition was observed to stop immediately.
Materials characterization
Dynamic light scattering (DLS) measurements were performed in triplicate on a custom-made Brookhaven Instruments ZetaPlus apparatus at a scattering angle of 90° and temperature of 25 °C. Nanoparticle concentrations were adjusted using DI H2O in order to get signal count rates between 150–400 kcps. Prior to DLS measurements, samples were bath sonicated for ∼2 minutes. The autocorrelation functions (ACFs) were analyzed with a CONTIN algorithm to determine size distributions by volume with the baseline set automatically. In order to verify the DLS technique, commercially available nanostars were analyzed by the DLS and seen to give similar trends in the intensity and volume distributions of size as the particles in the current study which is further discussed in the ESI.† The autocorrelation functions (ACFs) and the size distributions for both the nanourchins and the particles from the current study are shown in Table S1.†Low resolution transmission electron microscopy (TEM) imaging (FEI Tecnai Spirit BioTwin) was performed at an accelerating voltage of 80 kV. A drop of diluted nanocluster dispersion was put on a 400 mesh ultrathin carbon-coated copper TEM grid. Excess liquid was blotted with a tissue and the grid was allowed to dry in 30 inch Hg vacuum. Next, the samples on the grid were further washed by adding a drop of DI H2O on the grid and blotting it with a tissue and dried again. The grids were also run on a Hitachi S5500 SEM for obtaining SEM and STEM images. Particle sizing was conducted using the ImageJ software (NIH) for 100 particles.71 The sized particles were manually screened to ensure that only single particles were being sized and aggregates were excluded.
Thermogravimetric analysis was performed using a Perkin-Elmer TGA 7 under a nitrogen atmosphere at a gas flow rate of 20 mL min−1. Nanoparticle samples were dried to a powder in a low pressure environment at 30 in Hg vacuum. Samples were heated to 100 °C in the TGA instrument for 20 minutes to further remove moisture. The samples were then heated from 100 °C to 900 °C at a rate of 15 °C min−1, and then held at 900 °C for 30 minutes to burn off any organic material.
Measurements of Au concentrations in solution were obtained by first dissolving the nanoparticles in aqua regia. 0.25 mL HNO3 was added to a glass vial followed by 0.03 mL of sample solution. 0.75 mL of HCl was injected into the mixture and the samples were allowed to sit overnight (∼12 h) in a fume hood, during which time the solutions turned colorless. Samples were then diluted with DI H2O until Au concentration was estimated to be between 1 and 5 μg mL−1. The concentrations were then measured with a GBC 908AA flame atomic absorption spectrometer (GBC Scientific Equipment Pty Ltd) equipped with an air-acetylene flame furnace which was calibrated using a set of gold standard solutions at 1, 2, 4 and 5 μg mL−1. The absorption for Au was recorded at 242.8 nm to measure the concentration.
Results and discussion
Formation of clusters of Au primary particles
The nanoparticle synthesis was carried out at a Au3+ precursor concentration of 0.018 mM which is much lower than the typically used values of ∼1 mM or higher.25,35,72,73 At all three pH values studied (9.3, 8.7, and 7.5), initially ∼5 nm primary Au particles formed and then aggregated into small nanoclusters. A similar type of cluster formation has also been seen in other studies with semiconductor or metallic nanoparticles.58,59,72 The formation of the nanoclusters is evidenced by the appearance of blue color in the reaction solution, as quantified by the significant extinction in the >600 nm wavelength region of the UV-visible spectrum shown in Fig. 1a. Upon quenching the reaction after 40 s at pH 9.3 with mercaptoacetic acid, the morphology of these particles as seen in TEM micrographs in Fig. 1b was that of a nanocluster composed of several closely spaced primary particles. The coupling of the surface plasmon resonances (SPR) of the closely spaced primary particles along with the formation of some Au bridges between the primary particles possibly produces the large red-shift in the SPR.In order to further understand the formation of these clusters, it is instructive to consider recent mechanisms from the literature. Both thermodynamic and kinetic aspects contribute to the assembly of the primary particles into clusters to form the starting nanoclusters shown on the left-hand side of Scheme 1. From a thermodynamic point of view, clusters may be formed when short ranged attraction between primary particles causes growth of the aggregates until the net long ranged electrostatic repulsion between the charged primary particles in the cluster limits the cluster size.47,58,74–78 In the current study, the polymeric stabilizer is only weakly adsorbing and thus the van der Waals forces can cause the primary particles to aggregate. In summary, as the clusters grow larger, their size may be limited by the equilibrium between the net electrostatic repulsion from all the particles in the cluster. However, in this case, cluster formation and growth would not be expected to be solely equilibrium driven, but various mechanisms of kinetic control are expected to be present. For example, reduction of Au3+ precursor onto primary particles continues even as the primary particles aggregate (Fig. 1 and Scheme 1). The Au3+ precursor reduction may fuse the primary particles together, resulting in the formation of irreversible clusters as has been studied previously.2,35,69 Another kinetic aspect is passivation of the Au growth by adsorbed polymer on the nanocluster surface. Given the complexity of thermodynamic and kinetic aspects of the particle growth, it is instructive to attempt to regulate the kinetic growth rates with variables such as pH to better control the morphology and spectral properties.
Rapid kinetic growth generates protuberances on primaries whereas slow growth results in equilibrium relaxation to spheres
At the highest pH of 9.3, the Au3+ reduction rate is the slowest. Thus, the initial nanoclusters relax towards the thermodynamically favored state of large single spheres to minimize surface area/volume as shown in the upper part of Scheme 1. The reaction mixture was initially blue at 30 s, as described above, but then underwent a slow color change from blue at 2 min to purple at 2.5–3 min and eventually to pink in 3–4 min after which there was no further color change. Due to thermodynamic driving forces, the initial nanocluster is filled in progressively to form a sphere, as shown by the TEM micrographs in Fig. 1c–e, in order to minimize the surface area per volume. The spheres have a high extinction at 530 nm but very little in the NIR region as shown in Fig. 2a, as is expected for the SPR for spheres. The diameter of the resulting particles is determined to be ∼25 nm by dynamic light scattering (DLS) as shown in Fig. 2b. The size measured by DLS is in reasonable agreement with the average size of 34 nm measured for 100 particles from the TEM micrographs using the ImageJ software, with a histogram of the nanoparticle sizes shown in Fig. 2c.In contrast to the slow reaction at pH 9.3, more rapid growth occurs in the kinetic regime at lower pH values (8.7 and 7.5). The reaction mixture remained blue in color and darkened with time, as indicated by the absence of a peak shift in the UV-visible spectra in Fig. S1.† Additionally, the reaction occurred at a much faster rate, with the color change reaching the final state in 40 s at pH 8.7 and 25 s at pH 7.5 compared to 3–4 minutes for pH 9.3. The initial nanoclusters formed at the lower pH are similar in morphology and extinction properties (Fig. 1a and S1†) to the ones formed at pH 9.3, as shown by the TEM micrograph in Fig. S2a† (for pH 8.7). However, further growth at the lower pH conditions produces protuberances on these initial nanoclusters (Fig. S2b and S2c†) in contrast to the relaxation to equilibrium favored spheres at higher pH values. The particle size of the nanoparticles synthesized at pH 8.7 was ∼60 nm as measured by DLS (Fig. 2b), which agrees with the size determined by TEM as shown in the histogram in Fig. 2d. The measurement of the hydrodynamic diameter is useful not only for further characterizing the particles, but also for giving an indication of how the particles will behave in vivo. Here the small particles are decorated with a very high number of protuberances on a nanocluster core, as shown in TEM micrographs in Fig. 3a and S3.† In contrast, the growth is uncontrolled at pH 7.5 and results in very large particles with a diameter of 180 nm by DLS (Fig. 2b) and 150 nm from TEM micrographs (Fig. 2e). Here, the morphology is large star shaped particles as shown in the TEM micrographs in Fig. 3b.
The large number of protuberances on the particles with nanocluster cores synthesized at pH 8.7 and pH 7.5 produce high extinction in the NIR region out to 1100 nm with the peak absorbance at 650 nm as shown in Fig. 2a. Several factors influence the difference in the shape of the SPR at pH 8.7 and 7.5 that cannot be clearly delineated from the TEM. These include the spacing between primary particles, the size of the protuberances relative to the primary particle size and the size of the nanoparticles, the shape of the protuberances and finally the overall deviation in the shape of the nanoparticles from a spherical shape.
In contrast commercially available nanourchins with a similar diameter of 50 nm have a much lower NIR extinction with the peak at 576 nm and a sharp decline in extinction out to 1100 nm. The spectra for the particles in the current study extend out to 1100 nm as a consequence of the polydispersity in the particle size and shape. The remaining spectra in this manuscript are limited to 850 nm as they were measured with a Cary UV-vis spectrophotometer. While nanorods have spectra with significant absorbance at wavelength > 1100 nm, their absorbance is not as broad as for these nanoclusters. The high NIR absorbance is the result of plasmon hybridization for the protuberances on the spherical primary particles as well as the morphology of the nanocluster core.25,43 The difference in the extinction spectra of the particle dispersions is also evident visually in the photograph in Fig. 2f with the pink dispersion at pH 9.3 and the blue dispersions at pH 8.7 and 7.5. The NIR extinction properties of these particles which were synthesized with CMD as the stabilizing polymer are summarized in Table 1 along with the particle size by DLS. Furthermore, the particles are seen to have a highly negative zeta potential which arises from the adsorbed polymer on the surface (Table 1). The high zeta potential stabilizes the particles preventing them from aggregating. The particle size and spectra remained stable for up to a week (data not shown). Very similar morphologies were obtained for the particles synthesized using dextran in place of CMD (Fig. S4 and Table S2†), despite the fact that dextran is uncharged unlike CMD. Both CMD and dextran provided steric stabilization, and both were weakly bound to the Au surface given the loadings of 50 and 40% by weight respectively as seen from thermogravimetric analysis (TGA) in Fig. 4 and S5.†
| pH | Hydrodynamic diameter (nm) | Extinction ratio (800 nm/500 nm) | Zeta potential (mV) |
|---|---|---|---|
| 9.3 | 23 ± 4.2 | 0.034 | −33.8 ± 1.9 |
| 8.7 | 36 ± 6.3 | 1.24 | −33.9 ± 0.9 |
| 7.5 | 180 ± 30.4 | 1.31 | −35.8 ± 2.3 |
The Au3+ precursor (HAuCl4) varies as a function of the pH of the solution as seen in the data as a result of a previously described mechanism. The hydroxyl ions replace the chloride ions in the Au3+ complex, with the complex becoming less reactive when a higher fraction of chloride ligands have been replaced by hydroxyl ligands.2,72,79 At higher values of pH, the hydroxyl ion concentration is higher, which causes a shift in the equilibrium favoring increased replacement of chloride ions by hydroxyl ions according to le Chatelier's principle. Therefore as the pH of the reaction mixture increases, the Au3+ precursor becomes less reactive resulting in a slower reaction rate in agreement with our observation of the slowest reaction rate based on rate of color change at pH 9.3. The reaction rate is intermediate at pH 8.7 and fastest at pH 7.5, where the uncontrolled rapid growth forms particles that are too large for biomedical applications.
The kinetic and thermodynamic aspects of the particle formation mechanism are highly dependent upon pH. At the lowest pH values, where the reduction rate is the fastest, the reduction of Au3+ is primarily in the kinetically controlled growth regime. Here the reduced Au3+ precursor is added rapidly on the surface with limited time to develop a thermodynamically favored structure. In the current study, under kinetically-controlled growth at pH 8.7 and 7.5, protuberances are formed as shown in the bright field STEM images in Fig. 3a and b.
The behavior may be contrasted with kinetic growth on single monolithic particles that has been used to form a wide variety of non-equilibrium particle shapes. For nanostars (and nanourchins) the growth of protuberances on a monolithic core can only occur on certain crystal facets.25,29,36,80 With only a single core for growth on a monolithic particle, the number of available sites for growth of tips is limited. A similar phenomenon is seen in the growth of Pd shells on Au particles where Au particle cores with different shapes resulted in different Pd outer layer morphologies due to variation in the number of available facets for growth.81
In the current study, the protuberances are grown on a nanocluster consisting of numerous primary particles, each of which has multiple available growth sites. Thus, the growth on nanoclusters enables the formation of more protuberances per particle. In addition to the larger density of protuberances per particle, the resulting protuberances may also have a higher protuberance length-to-core diameter aspect ratio than comparable nanostar particles.3,20–22 The higher aspect ratio is a consequence of growth of protuberances on a 5–10 nm primary particle, relative to a larger core for the case of single monolithic cores as shown in the right parts of Scheme 1 for particles with a nanocluster core and Scheme 2 for monolithic cores. Therefore, kinetically-controlled growth of a large number of protuberances on our alternative type of core, namely, a nanocluster core, and with a high protuberance-to core ratio results in extraordinarily broad NIR extinction.
 | ||
| Scheme 2 Addition of more iterations of Au precursor to the reaction mixture at pH 9.3 leads to growth of protuberances on the spherical core. | ||
In order to produce sub 100 nm particles with strong NIR absorbance, it was necessary to prevent uncontrolled growth. Commonly the uncontrolled growth leads to particle sizes of 100–200 nm. To restrict growth, an unusually low Au3+ precursor concentration of 0.018 mM was utilized. To highlight the importance of the low Au3+ precursor concentration, the synthesis was carried out at pH 8.7 with a Au3+ precursor concentration of 0.036 mM, twice the normal concentration, while keeping all other parameters constant. Particles about twice as large were formed as shown by TEM images in Fig. 5a and by the hydrodynamic diameter from DLS in Fig. 5b. The significant increase in particle size is likely the result of the autocatalytic nature of the growth of Au on Au.57,82–84 Therefore at higher Au3+ concentrations of 1 mM or greater, the more rapid growth on the Au nuclei leads to the larger particles. Even larger particles have been observed in previous studies at even higher Au3+ precursor concentrations.29,35,85 Despite the near doubling of the nanoparticle size for the higher Au3+ concentration, the NIR extinction properties were not improved greatly over the particles synthesized with lower Au3+ concentrations as can be seen in Fig. 5c.
In contrast to the kinetically driven formation of protuberances at low pH values, the slower reactions at higher pH proceed in a thermodynamically controlled regime. Here, a Au3+ precursor has more time to diffuse to an energetically favorable site on the particle surface before being reduced. Consequently, the structure relaxed to a spherical shape as shown by the TEM images in Fig. 1b–d with the lowest possible surface area per volume.53,54
Formation of protuberances on the nanoclusters gives rise to high NIR extinction
It is instructive to contrast the properties of the particles synthesized in the current study with those of nanostars with monolithic cores. A totally symmetric sphere exhibits a SPR with a characteristic wavelength of 520 nm. The growth of a protuberance on a sphere produces a red shift in the resonant frequency of the surface plasmon through hybridization of the dipoles in the sphere and the protuberance to form multipoles.25,43 The cross-section or amplitude of the extinction is influenced by the core of the particle, but the shift in the frequency is caused solely by the protuberances.43 As a result, a larger core is capable of producing higher amplitude in the SPR. However, the particles would be quite large given an aspect ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of protuberance length to core radius required to produce a strong NIR shift. For typical monolithic nanostars with a relatively large core, the red-shift is produced primarily by the protuberances and not the monolithic particle cores.25,29 However, more work is necessary for developing models for these anisotropic particles to understand the effect of structure on SPR.86 In contrast, the initial nanocluster cores in the present study already produce extinction in the NIR (Fig. 1a), which is further enhanced by the formation of the protuberances. The proximity between the primary particles in the cluster interior results in plasmon hybridization between primary particles in the core, resulting in a red shift in the extinction peak.47,48,74,87–89
1 of protuberance length to core radius required to produce a strong NIR shift. For typical monolithic nanostars with a relatively large core, the red-shift is produced primarily by the protuberances and not the monolithic particle cores.25,29 However, more work is necessary for developing models for these anisotropic particles to understand the effect of structure on SPR.86 In contrast, the initial nanocluster cores in the present study already produce extinction in the NIR (Fig. 1a), which is further enhanced by the formation of the protuberances. The proximity between the primary particles in the cluster interior results in plasmon hybridization between primary particles in the core, resulting in a red shift in the extinction peak.47,48,74,87–89
For the particles synthesized at the lower pH values of 8.7 and 7.5, growth of protuberances further contributes to the red-shift in the SPR (Fig. 2a), beyond the contribution of the nanocluster cores (Fig. 1a). The surface density of protuberances, as explained earlier, is unusually high. As the number of protuberances per particle increases, a greater red shift is observed experimentally in the SPR.35,44,69 Additionally, as the protuberance length to core diameter aspect ratio increases, the red shift increases.25,43,44 In the current study, the aspect ratio is very large as the core is composed of small 5–10 nm primary particles, thus allowing a much higher shift in the SPR compared to particles with a monolithic core. The shift is caused by a combination of the aspect ratio of the protuberances to both the size of the primary particle to which it is connected and to the size of the nanocluster core. Therefore the particle morphology in the current study with the very high number of high aspect ratio protuberances per particle produces an unusually large shift in the characteristic plasmon frequency to the NIR region.
The broad extinction for these particles is highly unusual, for example, at 1000 nm the extinction is ∼80% of the value at the peak at ∼650 nm. This broad extinction is highly useful for applications including photoacoustic imaging.90 In previous studies, for similarly sized nanoparticles, this extinction ratio (1000 nm/650 nm) is below 30%.25,56 Typically Au nanoparticles must be much larger than 100 nm to achieve a similar level of NIR extinction.66,70 The polydispersity in spacings of the primary particles in the current study, along with the presence of the protuberances, provides the broader extinction. Additionally, the polydispersity in cluster size may also be essential to further broaden the extinction compared to other polydisperse25,70 or relatively monodisperse particles.56,91 At the current time, it appears that it is not possible to achieve broad NIR extinction without relatively high polydispersity in such small particles, although future optimization may overcome this limitation.
Multiple iterations of Au on spherical cores lead to conventional nanostars
To further place the results for the nanocluster cores containing a high density of protuberances in perspective, protuberances were also grown on the spherical particles synthesized at pH 9.3. The growth of protuberances was enabled by adding aliquots of Au3+ precursor (called iterations) which were rapidly reduced onto the particles. In this case, dextran was used as the stabilizing polymer instead of CMD to show that the technique has general applicability. Very similar results would be expected for CMD. The first iteration produced the same particles as in Fig. 1d and e as shown by the TEM image in Fig. 6a. As additional iterations of the Au3+ precursor were added, the growth of protuberances on the spheres was observed as shown in Scheme 2. Each additional iteration resulted in the elongation of the protuberances as shown in Fig. 6b–d. The growth of these protuberances is probably the result of a progressive decrease in the reaction mixture pH due to the addition of the acidic Au3+ precursor, which pushes the reaction into the kinetically-controlled growth regime and allows the growth of non-equilibrium protuberance structures on the particles. The growth of these protuberances increases the particle size as seen from the hydrodynamic diameter of the particle measured by DLS in Fig. 7a. As shown, the particle diameter is initially 25 nm and grows to 120 nm after 12 added iterations of Au3+ precursor.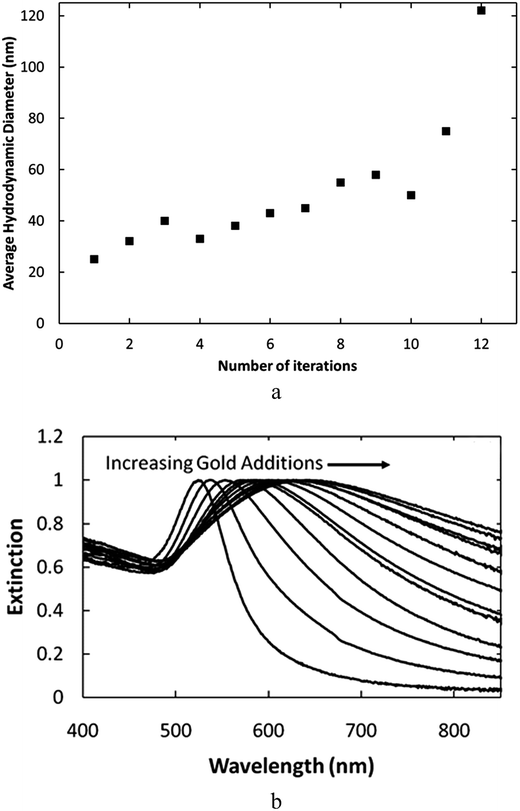 | ||
| Fig. 7 Nanoparticles after 1–12 iterations at 0.018 mM Au3+ per iteration with dextran coating at pH = 9.3 characterized by (a) DLS hydrodynamic diameter and (b) UV-Vis extinction spectra. | ||
The UV-vis extinction spectra for these particles shows that the plasmon peak shifts further to the red as more iterations of Au3+ precursor are added, as evidenced in Fig. 7b. The red-shift results from the growth of protuberances on the particles. As seen in earlier studies, the increased protuberance length to core diameter aspect ratio, due to the longer protuberances, increases the extent of the red shift.25,43,44 The intensity of NIR extinction for these particles, however, is much inferior to the particles synthesized at lower pH, which also exhibit asymmetric nanocluster cores (Fig. 2a). Therefore, the growth of protuberances on an asymmetric nanocluster core produces a greater NIR absorbance than growth of protuberances on a more conventional symmetric core at a similar size of ∼60 nm.
Conclusions
The growth of a high density of protuberances, on a nanocluster core substrate, results in particles with high NIR extinction up to wavelengths of 1100 nm, even for small diameters of ∼60 nm. These particles were formed at pH 8.7 and 7.5 in a more kinetically controlled regime, to avoid relaxation to spheres in the more thermodynamically controlled slow growth regime at higher pH. The large number of primary particles per cluster provided a greater number of reactive sites for growth of the protuberances, relative to the case of a monolithic core. The plasmon hybridization produces a broad NIR extinction as a result of several factors, namely, the close primary particle spacings in the initial nanocluster cores, the high density of protuberances on the cores, and the high protuberance length-to-primary particle diameter aspect ratio. In contrast, much larger protuberances are required for nanoparticles with spherical or monolithic cores (e.g. nanostars) to achieve the same NIR extinction resulting in much larger particles. The strong NIR extinction for a particle with a small size is of interest in biomedical imaging including photoacoustic imaging and photothermal therapy.Author contributions
AUB, BWW, AT, JJH, RJS, and KPJ designed and conducted the experiments. AUB, BWW, AT, TWW, TEM, TMT, and KPJ interpreted and discussed the data. AUB, BWW, JJH, TMT and KPJ wrote the manuscript. All authors have given approval to the final version of the manuscript.Funding sources
This work is supported in part by the Welch Foundation (F-1319 (KPJ), F-1696 (TMT)), NSF CBET-0968038, INSPIRE 1247945 and the Veterans Hospital Administration Merit Grant.Acknowledgements
This work is supported in part by the Welch Foundation (F-1319 (KPJ), F-1696 (TMT)), NSF CBET-0968038, INSPIRE 1247945 and the Veterans Hospital Administration Merit Grant. We would like to thank Dr Dwight Romanowicz from the Institute of Cellular and Molecular biology and Dr Shouliang Zhang from the Center for Nanomaterials both at the University of Texas for their assistance with the electron microscopy.Notes and references
- L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(23), 13549–13554 CrossRef CAS PubMed.
- L. L. Ma, M. D. Feldman, J. M. Tam, A. S. Paranjape, K. K. Cheruku, T. A. Larson, J. O. Tam, D. R. Ingram, V. Paramita, J. W. Villard, J. T. Jenkins, T. Wang, G. D. Clarke, R. Asmis, K. Sokolov, B. Chandrasekar, T. E. Milner and K. P. Johnston, Small Multifunctional Nanoclusters (Nanoroses) for Targeted Cellular Imaging and Therapy, ACS Nano, 2009, 3(9), 2686–2696 CrossRef CAS PubMed.
- J. Zhang, K. Sasaki, E. Sutter and R. R. Adzic, Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters, Science, 2007, 315(5809), 220–222 CrossRef CAS PubMed.
- V. E. Ferry, J. N. Munday and H. A. Atwater, Design considerations for plasmonic photovoltaics, Adv. Mater., 2010, 22(43), 4794–4808 CrossRef CAS.
- H. A. Atwater and A. Polman, Plasmonics for improved photovoltaic devices, Nat. Mater., 2010, 9(10), 865 CrossRef CAS , vol. 9, pg. 205, 2010.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. van Duyne, Biosensing with plasmonic nanosensors, Nat. Mater., 2008, 7(6), 442–453 CrossRef CAS PubMed.
- M. E. Stewart, C. R. Anderton, L. B. Thompson, J. Maria, S. K. Gray, J. A. Rogers and R. G. Nuzzo, Nanostructured plasmonic sensors, Chem. Rev., 2008, 108(2), 494–521 CrossRef CAS PubMed.
- R. Weissleder, A clearer vision for in vivo imaging, Nat. Biotechnol., 2001, 19(4), 316–317 CrossRef CAS PubMed.
- M. E. Kooi, V. C. Cappendijk, K. Cleutjens, A. G. H. Kessels, P. Kitslaar, M. Borgers, P. M. Frederik, M. Daemen and J. M. A. van Engelshoven, Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging, Circulation, 2003, 107(19), 2453–2458 CrossRef CAS.
- Y. Ma, N. Li, C. Yang and X. R. Yang, One-step synthesis of amino-dextran-protected gold and silver nanoparticles and its application in biosensors, Anal. Bioanal. Chem., 2005, 382(4), 1044–1048 CrossRef CAS PubMed.
- M. Arruebo, R. Fernandez-Pacheco, M. R. Ibarra and J. Santamaria, Magnetic nanoparticles for drug delivery, Nano Today, 2007, 2(3), 22–32 CrossRef.
- T. Betancourt, B. Brown and L. Brannon-Peppas, Doxorubicin-loaded PLGA nanoparticles by nanoprecipitation: preparation, characterization and in vitro evaluation, Nanomedicine, 2007, 2(2), 219–232 CrossRef CAS PubMed.
- M. L. Schipper, G. Iyer, A. L. Koh, Z. Cheng, Y. Ebenstein, A. Aharoni, S. Keren, L. A. Bentolila, J. Q. Li, J. H. Rao, X. Y. Chen, U. Banin, A. M. Wu, R. Sinclair, S. Weiss and S. S. Gambhir, Particle Size, Surface Coating, and PEGylation Influence the Biodistribution of Quantum Dots in Living Mice, Small, 2009, 5(1), 126–134 CrossRef CAS PubMed.
- M. E. Davis, Z. Chen and D. M. Shin, Nanoparticle therapeutics: an emerging treatment modality for cancer, Nat. Rev. Drug Discovery, 2008, 7(9), 771–782 CrossRef CAS.
- M. C. Daniel and D. Astruc, Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology, Chem. Rev., 2004, 104(1), 293–346 CrossRef CAS.
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods, J. Am. Chem. Soc., 2006, 128(6), 2115–2120 CrossRef CAS.
- N. J. Durr, T. Larson, D. K. Smith, B. A. Korgel, K. Sokolov and A. Ben-Yakar, Two-photon luminescence imaging of cancer cells using molecularly targeted gold nanorods, Nano Lett., 2007, 7(4), 941–945 CrossRef CAS PubMed.
- D. Pissuwan, S. M. Valenzuela, M. C. Killingsworth, X. D. Xu and M. B. Cortie, Targeted destruction of murine macrophage cells with bioconjugated gold nanorods, J. Nanopart. Res., 2007, 9(6), 1109–1124 CrossRef CAS.
- L. Qiu, T. A. Larson, D. K. Smith, E. Vitkin, S. H. Zhang, M. D. Modell, I. Itzkan, E. B. Hanlon, B. A. Korgel, K. V. Sokolov and L. T. Perelman, Single gold nanorod detection using confocal light absorption and scattering spectroscopy, IEEE J. Sel. Top. Quantum Electron., 2007, 13(6), 1730–1738 CrossRef CAS.
- J. Zhu, K. T. Yong, I. Roy, R. Hu, H. Ding, L. L. Zhao, M. T. Swihart, G. S. He, Y. P. Cui and P. N. Prasad, Additive controlled synthesis of gold nanorods (GNRs) for two-photon luminescence imaging of cancer cells, Nanotechnology, 2010, 21, 285106 CrossRef PubMed.
- D. C. Adler, S. W. Huang, R. Huber and J. G. Fujimoto, Photothermal detection of gold nanoparticles using phase-sensitive optical coherence tomography, Opt. Express, 2008, 16(7), 4376–4393 CrossRef CAS.
- C. Loo, A. Lowery, N. J. Halas, J. West and R. Drezek, Immunotargeted nanoshells for integrated cancer imaging and therapy, Nano Lett., 2005, 5(4), 709–711 CrossRef CAS PubMed.
- M. R. Langille, M. L. Personick, J. Zhang and C. A. Mirkin, Bottom-Up Synthesis of Gold Octahedra with Tailorable Hollow Features, J. Am. Chem. Soc., 2011, 133(27), 10414–10417 CrossRef CAS PubMed.
- S. E. Skrabalak, J. Chen, L. Au, X. Lu, X. Li and Y. Xia, Gold nanocages for biomedical applications, Adv. Mater., 2007, 19(20), 3177–3184 CrossRef CAS.
- S. Barbosa, A. Agrawal, L. Rodriguez-Lorenzo, I. Pastoriza-Santos, R. A. Alvarez-Puebla, A. Kornowski, H. Weller and L. M. Liz-Marzan, Tuning Size and Sensing Properties in Colloidal Gold Nanostars, Langmuir, 2010, 26(18), 14943–14950 CrossRef CAS PubMed.
- C. L. Nehl, H. W. Liao and J. H. Hafner, Optical properties of star-shaped gold nanoparticles, Nano Lett., 2006, 6(4), 683–688 CrossRef CAS PubMed.
- D. Senapati, A. K. Singh and P. C. Ray, Real time monitoring of the shape evolution of branched gold nanostructure, Chem. Phys. Lett., 2010, 487(1–3), 88–91 CrossRef CAS.
- M. Mueller, M. Tebbe, D. V. Andreeva, M. Karg, R. A. Alvarez Puebla, N. Pazos Perez and A. Fery, Large-area organization of pNIPAM-coated nanostars as SERS platforms for polycyclic aromatic hydrocarbons sensing in gas phase, Langmuir, 2012, 28(24), 9168–9173 CrossRef CAS PubMed.
- M. Grzelczak and L. M. Liz-Marzán, Colloidal Nanoplasmonics: From Building Blocks to Sensing Devices, Langmuir, 2013, 29(15), 4652–4663 CrossRef CAS PubMed.
- A. Casu, E. Cabrini, A. Donà, A. Falqui, Y. Diaz-Fernandez, C. Milanese, A. Taglietti and P. Pallavicini, Controlled Synthesis of Gold Nanostars by Using a Zwitterionic Surfactant, Chem.–Eur. J., 2012, 18(30), 9381–9390 CrossRef CAS.
- A. Kedia and P. S. Kumar, Controlled reshaping and plasmon tuning mechanism of gold nanostars, J. Mater. Chem. C, 2013, 1(30), 4540 RSC.
- P. Pallavicini, A. Donà, A. Casu, G. Chirico, M. Collini, G. Dacarro, A. Falqui, C. Milanese, L. Sironi and A. Taglietti, Triton X-100 for three-plasmon gold nanostars with two photothermally active NIR (near IR) and SWIR (short-wavelength IR) channels, Chem. Commun., 2013, 49(56), 6265 RSC.
- Q. Su, X. Ma, J. Dong, C. Jiang and W. Qian, A Reproducible SERS Substrate Based on Electrostatically Assisted APTES-Functionalized Surface-Assembly of Gold Nanostars, ACS Appl. Mater. Interfaces, 2011, 3(6), 1873–1879 CAS.
- Z. D. Wang, J. Q. Zhang, J. M. Ekman, P. J. A. Kenis and Y. Lu, DNA-Mediated Control of Metal Nanoparticle Shape: One-Pot Synthesis and Cellular Uptake of Highly Stable and Functional Gold Nanoflowers, Nano Lett., 2010, 10(5), 1886–1891 CrossRef CAS PubMed.
- T. T. Nhung, Y. Bu and S.-W. Lee, Facile synthesis of chitosan-mediated gold nanoflowers as surface-enhanced Raman scattering (SERS) substrates, J. Cryst. Growth, 2013, 373, 132–137 CrossRef CAS.
- S. Yi, L. Sun, S. C. Lenaghan, Y. Wang, X. Chong, Z. Zhang and M. Zhang, One-step synthesis of dendritic gold nanoflowers with high surface-enhanced Raman scattering (SERS) properties, RSC Adv., 2013, 3(26), 10139 RSC.
- L. H. Lu, K. Ai and Y. Ozaki, Environmentally friendly synthesis of highly monodisperse biocompatible gold nanoparticles with urchin-like shape, Langmuir, 2008, 24(3), 1058–1063 CrossRef CAS PubMed.
- H. Yuan, W. Ma, C. Chen, J. Zhao, J. Liu, H. Zhu and X. Gao, Shape and SPR Evolution of Thorny Gold Nanoparticles Promoted by Silver Ions, Chem. Mater., 2007, 19(7), 1592–1600 CrossRef CAS.
- C. H. Kuo and M. H. Huang, Synthesis of branched gold nanocrystals by a seeding growth approach, Langmuir, 2005, 21(5), 2012–2016 CrossRef CAS PubMed.
- X. Q. Zou, E. B. Ying and S. J. Dong, Seed-mediated synthesis of branched gold nanoparticles with the assistance of citrate and their surface-enhanced Raman scattering properties, Nanotechnology, 2006, 17(18), 4758–4764 CrossRef CAS PubMed.
- B. van de Broek, F. Frederix, K. Bonroy, H. Jans, K. Jans, G. Borghs and G. Maes, Shape-controlled synthesis of NIR absorbing branched gold nanoparticles and morphology stabilization with alkanethiols, Nanotechnology, 2011, 22, 015601 CrossRef CAS PubMed.
- H.-M. Song, Q. Wei, Q. K. Ong and A. Wei, Plasmon-Resonant Nanoparticles and Nanostars with Magnetic Cores: Synthesis and Magnetomotive Imaging, ACS Nano, 2010, 4(9), 5163–5173 CrossRef CAS PubMed.
- L. Stagg, S.-Q. Zhang, M. S. Cheung and P. Wittung-Stafshede, Molecular crowding enhances native structure and stability of alpha/beta protein flavodoxin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(48), 18976–18981 CrossRef CAS PubMed.
- P. Senthil Kumar, I. Pastoriza-Santos, B. Rodriguez-Gonzalez, F. Javier Garcia de Abajo and L. M. Liz-Marzan, High-yield synthesis and optical response of gold nanostars, Nanotechnology, 2008, 19(1), 015606 CrossRef PubMed.
- J. M. Tam, A. K. Murthy, D. R. Ingram, R. Nguyen, K. V. Sokolov and K. P. Johnston, Kinetic Assembly of Near-IR-Active Gold Nanoclusters Using Weakly Adsorbing Polymers to Control the Size, Langmuir, 2010, 26(11), 8988–8999 CrossRef CAS PubMed.
- J. M. Tam, J. O. Tam, A. Murthy, D. R. Ingram, L. L. Ma, K. Travis, K. P. Johnston and K. V. Sokolov, Controlled Assembly of Biodegradable Plasmonic Nanoclusters for Near-Infrared Imaging and Therapeutic Applications, ACS Nano, 2010, 4(4), 2178–2184 CrossRef CAS.
- A. K. Murthy, R. J. Stover, A. U. Borwankar, G. D. Nie, S. Gourisankar, T. M. Truskett, K. V. Sokolov and K. P. Johnston, Equilibrium Gold Nanoclusters Quenched with Biodegradable Polymers, ACS Nano, 2013, 7(1), 239–251 CrossRef CAS PubMed.
- N. Pazos-Perez, F. J. Garcia de Abajo, A. Fery and R. A. Alvarez-Puebla, From nano to micro: synthesis and optical properties of homogeneous spheroidal gold particles and their superlattices, Langmuir, 2012, 28(24), 8909–8914 CrossRef CAS PubMed.
- N. Pazos-Perez, C. S. Wagner, J. M. Romo-Herrera, L. M. Liz-Marzan, F. J. Garcia de Abajo, A. Wittemann, A. Fery and R. A. Alvarez-Puebla, Organized plasmonic clusters with high coordination number and extraordinary enhancement in surface-enhanced Raman scattering (SERS), Angew. Chem., Int. Ed. Engl., 2012, 51(51), 12688–12693 CrossRef CAS PubMed.
- Y. Zhao, L. Xu, L. M. Liz-Marzán, H. Kuang, W. Ma, A. Asenjo-García, F. J. García de Abajo, N. A. Kotov, L. Wang and C. Xu, Alternating Plasmonic Nanoparticle Heterochains Made by Polymerase Chain Reaction and Their Optical Properties, J. Phys. Chem. Lett., 2013, 4(4), 641–647 CrossRef CAS PubMed.
- B. Luk'yanchuk, N. I. Zheludev, S. A. Maier, N. J. Halas, P. Nordlander, H. Giessen and C. T. Chong, The Fano resonance in plasmonic nanostructures and metamaterials, Nat. Mater., 2010, 9(9), 707–715 CrossRef PubMed.
- B. L. Frankamp, A. K. Boal and V. M. Rotello, Controlled interparticle spacing through self-assembly of Au nanoparticles and poly(amidoamine) dendrimers, J. Am. Chem. Soc., 2002, 124(51), 15146–15147 CrossRef CAS PubMed.
- M. R. Langille, M. L. Personick, J. Zhang and C. A. Mirkin, Defining rules for the shape evolution of gold nanoparticles, J. Am. Chem. Soc., 2012, 134(35), 14542–14554 CrossRef CAS PubMed.
- M. R. Langille, J. Zhang, M. L. Personick, S. Li and C. A. Mirkin, Stepwise Evolution of Spherical Seeds into 20-Fold Twinned Icosahedra, Science, 2012, 337(6097), 954–957 CrossRef CAS PubMed.
- J. Chen, F. Saeki, B. J. Wiley, H. Cang, M. J. Cobb, Z.-Y. Li, L. Au, H. Zhang, M. B. Kimmey, X. D. Li and Y. N. Xia, Gold Nanocages: Bioconjugation and Their Potential Use as Optical Imaging Contrast Agents, Nano Lett., 2005, 5(3), 473–477 CrossRef CAS.
- Y. Kim do, T. Yu, E. C. Cho, Y. Ma, O. O. Park and Y. Xia, Synthesis of gold nano-hexapods with controllable arm lengths and their tunable optical properties, Angew. Chem., 2011, 50(28), 6328–6331 CrossRef PubMed.
- L. L. Ma, A. U. Borwankar, B. W. Willsey, K. Y. Yoon, J. O. Tam, K. V. Sokolov, M. D. Feldman, T. E. Milner and K. P. Johnston, Growth of textured thin Au coatings on iron oxide nanoparticles with near infrared absorbance, Nanotechnology, 2013, 24, 025606 CrossRef CAS PubMed.
- Y. Xia, T. D. Nguyen, M. Yang, B. Lee, A. Santos, P. Podsiadlo, Z. Tang, S. C. Glotzer and N. A. Kotov, Self-assembly of self-limiting monodisperse supraparticles from polydisperse nanoparticles, Nat. Nanotechnol., 2011, 6(9), 580–587 CrossRef CAS PubMed.
- Z. Lu, M. Ye, N. Li, W. Zhong and Y. Yin, Self-Assembled TiO2 Nanocrystal Clusters for Selective Enrichment of Intact Phosphorylated Proteins, Angew. Chem., Int. Ed., 2010, 49(10), 1862–1866 CrossRef CAS PubMed.
- A. S. D. S. Indrasekara, R. Thomas and L. Fabris, Plasmonic properties of regiospecific core-satellite assemblies of gold nanostars and nanospheres, Phys. Chem. Chem. Phys., 2015, 17(33), 21133–21142 RSC.
- M. Grzelczak, S. A. Mezzasalma, W. Ni, Y. Herasimenka, L. Feruglio, T. Montini, J. Pérez-Juste, P. Fornasiero, M. Prato and L. M. Liz-Marzán, Antibonding Plasmon Modes in Colloidal Gold Nanorod Clusters, Langmuir, 2012, 28(24), 8826–8833 CrossRef CAS PubMed.
- M. Grzelczak, A. Sánchez-Iglesias, H. H. Mezerji, S. Bals, J. Pérez-Juste and L. M. Liz-Marzán, Steric Hindrance Induces crosslike Self-Assembly of Gold Nanodumbbells, Nano Lett., 2012, 12(8), 4380–4384 CrossRef CAS PubMed.
- L. L. Zhao, X. H. Ji, X. J. Sun, J. Li, W. S. Yang and X. G. Peng, Formation and Stability of Gold Nanoflowers by the Seeding Approach: The Effect of Intraparticle Ripening, J. Phys. Chem. C, 2009, 113(38), 16645–16651 CAS.
- G. Marcelo and M. Fernandez-Garcia, Direct preparation of PNIPAM coating gold nanoparticles by catechol redox and surface adhesion chemistry, RSC Adv., 2014, 4(23), 11740–11749 RSC.
- L. Minati, F. Benetti, A. Chiappini and G. Speranza, One-step synthesis of star-shaped gold nanoparticles, Colloids Surf., A, 2014, 441(0), 623–628 CrossRef CAS.
- S. M. Novikov, A. Sánchez-Iglesias, M. K. Schmidt, A. Chuvilin, J. Aizpurua, M. Grzelczak and L. M. Liz-Marzán, Gold Spiky Nanodumbbells: Anisotropy in Gold Nanostars, Part. Part. Syst. Charact., 2014, 31(1), 77–80 CrossRef CAS.
- S. Trigari, A. Rindi, G. Margheri, S. Sottini, G. Dellepiane and E. Giorgetti, Synthesis and modelling of gold nanostars with tunable morphology and extinction spectrum, J. Mater. Chem., 2011, 21(18), 6531–6540 RSC.
- J. P. Wilcoxon, J. E. Martin and D. W. Schaefer, Aggregation in Colloidal Gold, Phys. Rev. A: At., Mol., Opt. Phys., 1989, 39(5), 2675–2688 CrossRef CAS.
- J. Xie, Q. Zhang, J. Y. Lee and D. I. C. Wang, The Synthesis of SERS-Active Gold Nanoflower Tags for In vivo Applications, ACS Nano, 2008, 2(12), 2473–2480 CrossRef CAS PubMed.
- A. Kedia and P. S. Kumar, Halide ion induced tuning and self-organization of gold nanostars, RSC Adv., 2014, 4(9), 4782–4790 RSC.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis, Nat. Methods, 2012, 9(7), 671–675 CrossRef CAS PubMed.
- D. V. Goia and E. Matijevic, Tailoring the particle size of monodispersed colloidal gold, Colloids Surf., A, 1999, 146(1–3), 139–152 CrossRef CAS.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system, J. Chem. Soc., Chem. Commun., 1994,(7), 801 RSC.
- A. K. Murthy, R. J. Stover, W. G. Hardin, R. Schramm, G. D. Nie, S. Gourisankar, T. M. Truskett, K. V. Sokolov and K. P. Johnston, Charged gold nanoparticles with essentially zero serum protein adsorption in undiluted fetal bovine serum, J. Am. Chem. Soc., 2013, 135(21), 7799–7802 CrossRef CAS PubMed.
- Z. Lu, C. Gao, Q. Zhang, M. Chi, J. Y. Howe and Y. Yin, Direct assembly of hydrophobic nanoparticles to multifunctional structures, Nano Lett., 2011, 11(8), 3404–3412 CrossRef CAS.
- K. P. Johnston, J. A. Maynard, T. M. Truskett, A. Borwankar, M. A. Miller, B. Wilson, A. K. Dinin, T. A. Khan and K. J. Kaczorowski, Concentrated Dispersions of Equilibrium Protein Nanoclusters That Reversibly Dissociate into Active Monomers, ACS Nano, 2012, 6, 21357–21369 Search PubMed.
- A. U. Borwankar, A. K. Dinin, J. R. Laber, A. Twu, B. K. Wilson, J. A. Maynard, T. M. Truskett and K. P. Johnston, Tunable equilibrium nanocluster dispersions at high protein concentrations, Soft Matter, 2013, 9(6), 1766 RSC.
- J. Groenewold and W. K. Kegel, Anomalously Large Equilibrium Clusters of Colloids, J. Phys. Chem. B, 2001, 105(47), 11702–11709 CrossRef CAS.
- T. Arakawa, D. Ejima, K. Tsumoto, N. Obeyama, Y. Tanaka, Y. Kita and S. N. Timasheff, Suppression of protein interactions by arginine: a proposed mechanism of the arginine effects, Biophys. Chem., 2007, 127(1–2), 1–8 CrossRef CAS PubMed.
- E. Giorgetti, S. Trigari, A. Rindi, G. Margheri, S. Sottini, G. Dellepiane, G. Brusatin, L. Brigo, M. Muniz-Miranda and I. Timtcheva, Tunable gold nanostars for surface enhanced Raman spectroscopy, Phys. Status Solidi B, 2012, 249(6), 1188–1192 CrossRef CAS.
- K. L. Jungjohann, S. Bliznakov, P. W. Sutter, E. A. Stach and E. A. Sutter, In situ liquid cell electron microscopy of the solution growth of Au–Pd core–shell nanostructures, Nano Lett., 2013, 13(6), 2964–2970 CrossRef CAS PubMed.
- L. Minati, F. Benetti, A. Chiappini and G. Speranza, One-step synthesis of star-shaped gold nanoparticles, Colloids Surf., A, 2014, 441, 623–628 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Evidence for seed-mediated nucleation in the chemical reduction of gold salts to gold nanoparticles, Chem. Mater., 2001, 13(7), 2313–2322 CrossRef CAS.
- M. A. Watzky and R. G. Finke, Transition Metal Nanocluster Formation Kinetic and Mechanistic Studies. A New Mechanism When Hydrogen Is the Reductant: Slow, Continuous Nucleation and Fast Autocatalytic Surface Growth, J. Am. Chem. Soc., 1997, 119(43), 10382–10400 CrossRef CAS.
- J. Xie, J. Y. Lee and D. I. C. Wang, Seedless, Surfactantless, High-Yield Synthesis of Branched Gold Nanocrystals in HEPES Buffer Solution, Chem. Mater., 2007, 19(11), 2823–2830 CrossRef CAS.
- T. W. Odom and C. L. Nehl, How Gold Nanoparticles Have Stayed in the Light: The 3M's Principle, ACS Nano, 2008, 2(4), 612–616 CrossRef CAS PubMed.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, The golden age: gold nanoparticles for biomedicine, Chem. Soc. Rev., 2012, 41(7), 2740–2779 RSC.
- N. J. Halas, S. Lal, W.-S. Chang, S. Link and P. Nordlander, Plasmons in Strongly Coupled Metallic Nanostructures, Chem. Rev., 2011, 111, 3913–3961 CrossRef CAS PubMed.
- N. G. Khlebtsov, L. A. Dykman, Y. M. Krasnov and A. G. Mel'nikov, Light Absorption by the Clusters of Colloidal Gold and Silver Particles Formed During Slow and Fast Aggregation, Colloid J., 2000, 62(6), 765–779 CrossRef CAS.
- S. J. Yoon, S. Mallidi, J. M. Tam, J. O. Tam, A. Murthy, K. P. Johnston, K. V. Sokolov and S. Y. Emelianov, Utility of biodegradable plasmonic nanoclusters in photoacoustic imaging, Opt. Lett., 2010, 35(22), 3751–3753 CrossRef CAS PubMed.
- M. R. Langille, M. L. Personick, J. Zhang and C. A. Mirkin, Defining Rules for the Shape Evolution of Gold Nanoparticles, J. Am. Chem. Soc., 2012, 134(35), 14542–14554 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis spectra and TEM images for quenching of particles at pH 8.7, additional TEM images of particles contained in the paper, TGA data for particles synthesized with dextran, UV-Vis spectra, sizes by DLS and TEM images of particles at pH 8.7 and 7.5 after additional Au deposition, ACF data, size distributions for DLS data presented in the paper and discussion of the DLS data. See DOI: 10.1039/c5ra21712a |
| This journal is © The Royal Society of Chemistry 2015 |






