Crystal structures and luminescent properties of new lanthanide(iii) complexes derived from 2-phenyl-4-pyrimidinecarboxylate†
Abstract
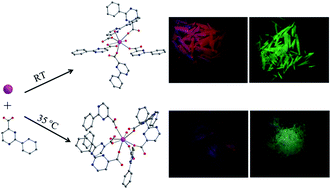
In this work, five novel lanthanide(III) coordination polymers derived from 2-phenylpyrimidine-4-carboxylic acid (Hppmc), namely, [Ln(ppmc)3(H2O)2]·2H2O [Ln = Eu (1), Tb (2)] and [Ln(ppmc)3(H2O)2] [Ln = Eu (3), Gd (4), Tb (5)] were successfully synthesized by a facile solution method and characterized by single-crystal X-ray diffraction, power X-ray diffraction (PXRD), infrared (IR) spectroscopy, elemental analysis, and thermogravimetric analysis (TGA). It was found that subtly different reaction conditions result in disparate structural characteristics. For example, by combining Hppmc with lanthanide(III) ions at room temperature, compounds 1 and 2 featuring a carboxylate-bridging chain structure, in which the carboxylates adopt both chelating and bridging modes, are isolated. However, the reaction at 35 °C generates three isostructural compounds 3–5 with a distinct chain structure, in which the lanthanide ions are connected by carboxylates via syn–syn and syn–anti modes. Photoluminescent studies of the Eu3+ and Tb3+ complexes reveal that the Hppmc ligand is a better sensitizer for Tb3+ ion than for Eu3+ ion. The investigation of the relationship between the crystal structures and the photoluminescence properties indicate that the coordination environments of lanthanide ions and the arrangement of the ligands are the dominating factors that affect the luminescence behaviors of the solid samples.

 Please wait while we load your content...
Please wait while we load your content...