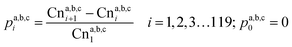Anaerobic co-digestion of municipal wastewater sludge with food waste with different fat, oil, and grease contents: study of reactor performance and extracellular polymeric substances†
Rui Xuab,
Zhaohui Yang*ab,
Ting Chenab,
Lijun Zhaoab,
Jing Huangab,
Haiyin Xuab,
Peipei Songab and
Min Liab
aCollege of Environmental Science and Engineering, Hunan University, Changsha 410082, P. R. China. E-mail: yzh@hnu.edu.cn; Fax: +86-731-88822829; Tel: +86-731-88822829
bKey Laboratory of Environmental Biology and Pollution Control (Hunan University), Ministry of Education, Changsha 410082, P. R. China
First published on 9th November 2015
Abstract
The linkage between reactor performance and microbial extracellular polymeric substances (EPS) was investigated in three groups of semi-continuous mesophilic anaerobic co-digestion (ACoD) systems, treating municipal waste sludge (MWS) with food waste (FW) with different fat, oil and grease (FOG) contents. The addition of FOG to the test reactors enhanced the co-digestion process significantly in terms of reactor performance and microbial activity. During the process, no major variations in pH and VFA/Alk were observed. Moreover, the daily yield of biogas peaked at 810.3 mL per g VSadded when the FOG load reached 42% of volatile solids (VS), with an organic loading rate (OLR) of 5.2 g VS L−1 d−1 and a hydraulic retention time (HRT) of 20 days. However, an excessive FOG load (55% of VS) reduced biogas production by 40.3% when compared with the control unit (539.3 mL per g VSadded). At the end of digestion, 195 L, 381 L and 351 L cumulative biogas were obtained in the three systems, respectively. Further analysis of extracellular polymeric substances (EPS) showed that the accumulation peaked at 648.5, 772.3 and 640.9 mg L−1 with the optimal digestion parameters, respectively. The proportion of LB-EPS was always less than that of TB-EPS, which accounted for about 40% and 60%. The FOG-enhanced systems (R2 and R3) produced considerably higher levels of EPS than the control system (R1) for both humic acid substances (HS) and proteins (PN). Moreover, variations in EPS revealed that the three systems experienced an accommodation phase followed by a vigorous phase and an exhausted phase with elevated levels of added FOG. However, enhanced units may undergo exhaustion prematurely due to “doping” phenomena.
1. Introduction
With the rapid development of the construction of cities, increasing numbers of wastewater treatment plants (WWTPs) have been set up and come into service for decades in China. As the main residue discharged from WWTPs, large amounts (30 million tons per annum) of municipal waste sludge (MWS) create a significant threat to public health and the environment when inappropriately disposed.1,2 Anaerobic digestion (AD) has been evaluated as a promising biological technology to alleviate the problem of sludge disposal, because it converts organics in MWS into a renewable bioenergy resource in the form of methane.3,4 AD could have the simultaneous benefits of a reduction in sludge volume, renewable energy recovery, dilution of potentially hazardous compounds and control of odor emissions, when compared with conventional sludge disposal procedures.5,6 However, employing MWS as the sole digestion substrate has limited the successful implementation of this approach due to the low C/N ratio contained in sewage sludge. This ratio, which is of the order of 6–16, is also regarded as a serious problem in anaerobic digestion.7 It should range from 20 to 30 to ensure a sufficient supply of nitrogen for cell production and degradation of the carbon present in the process.8Therefore, a readily available high-organic-content and highly biodegradable waste such as food waste (FW) is recognized as a desirable co-substrate material. Anaerobic co-digestion (ACoD) may substantially increase biogas yield due to the presence of abundant fat, oil, and grease (FOG) in FW.6 Furthermore, FOG has frequently been stated to effectively increase biogas production by 30% or more when directly added to an anaerobic digester based on its theoretical methane potential (1430 mL per g VSadded).9,10 Previous studies discussed concerns about inhibition or the potential for inhibition during ACoD. In fact, high FOG contents of organic wastes can lead to the accumulation of inhibitory compounds such as long-chain fatty acids (LCFAs), resulting in disturbances to the process by affecting the microbial composition.11,12 LCFAs, which are the primary component of FOG present in FW, are degraded anaerobically via the β-oxidation pathway to acetate and H2, which are subsequently converted into methane. β-Oxidation begins when fatty acids are activated by coenzyme A and subsequent oxidation leads to the release of acetyl CoA and the formation of a fatty acid chain.13 One concern is that LCFAs may have a detrimental effect on methanogenic bacteria when introduced at sufficiently high concentrations or loading rates. Researchers have suggested that the detrimental effect on methanogenic bacteria may be due to: sludge flotation and washout; transport limitations due to bacteria being coated by a layer of LCFAs, thereby reducing the cells' access to substrates and ability to release biogas; or a toxic effect of LCFAs on methanogenic bacteria. Digester foaming is another operational concern associated with anaerobic digestion of lipids.3 Foaming can result in inefficient gas recovery, an inverse solids profile with higher concentrations of solids at the top of a digester, blockages of gas mixing devices, and fouling of gas collection pipes.14 Therefore, it is crucial to reach a practicable compromise between waste treatment capacity and biogas yield without causing operational instability.
AD occurs via four main steps, namely, hydrolysis, acidogenesis, acetogenesis and methanogenesis,15 which rely on different microbial communities with all species living together in symbiotic associations. Therefore, it is necessary to determine the different digestion parameters that influence communities and optimize microbial activity. However, as most previous research has focused on the diversity and dynamics of microbial communities, little information is available to specifically address the role of microbial metabolism in the digestion process. Extracellular polymeric substances (EPS) in biological sludge treatment systems are a general feature of microbial communities. The production and composition of EPS mainly arise from active bacterial secretion, shedding of cell surface material, cell lysis and desorption from the surface of an external matrix.16 EPS are composed of a variety of organic substances, including carbohydrates and proteins, as the major constituents and humic substances, uronic acids and nucleic acids in smaller quantities.17 It is notable that EPS partly result from microbial metabolism and therefore is affected by the composition and activity of a microbial community.18 In addition, recent suggestions have identified parameters, such as oil, grease, volatile fatty acids, detergents, proteins and products (EPS) of the metabolic activity of microorganisms, as causes of foaming in anaerobic digestion.19 Better insight into the degradation pathways and by-products of these compounds during ACoD could provide additional information on the relationship between microbial response and digestion performance. Therefore, it is worth investigating whether a FOG-enhanced ACoD system affects the accumulation of EPS.
This study investigated the influence of different FOG contents on ACoD of MWS and FW systems. The stability and general performance of the digestion process are discussed separately. The characteristics of microbial metabolism are discussed in terms of EPS by detecting polysaccharides, proteins, humic acid substances and nucleic acid components. Further investigation is a matter of great interest not only in terms of deepening the understanding of biological sludge treatment, but also in improving the efficiency of such treatment via the optimization of operational parameters.
2. Materials and methods
2.1 Inoculum and substrates preparation
Primary sludge and MWS were collected from a WWTP in Changsha, China. This plant is located in the Xiang River region and annually disposes 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of wastewater (90% domestic and 10% industrial sewage) by an oxidation ditch process. Raw food waste (RFW) was collected over 5 consecutive working days from a typical Chinese restaurant in Changsha, China. The collected samples were transported to the laboratory within 1 h and stored at 4 °C for no more than 3 days.
000 tons of wastewater (90% domestic and 10% industrial sewage) by an oxidation ditch process. Raw food waste (RFW) was collected over 5 consecutive working days from a typical Chinese restaurant in Changsha, China. The collected samples were transported to the laboratory within 1 h and stored at 4 °C for no more than 3 days.
Prior to pumping into the digestion reactors, (i) primary sludge was thickened by gravity for 6 h at 4 °C with the decantation of supernatant. The sediment seed sludge (SS) was sealed in a glass bottle with crimped butyl rubber stoppers and purged with nitrogen for 2 min to create anaerobic conditions. The bottle was subsequently incubated at 37 °C in a shaking incubator at 100 rpm for 15 days as an anaerobic adaptation period. (ii) RFW was squeezed thoroughly by a cement compressor (YAW-300C, Hengda), followed by a Soxhlet extraction method20 to eliminate FOG. (iii) MWS and FW were smashed and pasted, respectively, using an electric food grinder (XTL-767, IFAVORITE). (iv) Post-treated MWS, FW and FOG were stored at 4 °C until utilization. All of them were brought to room temperature before being added to the digester. The characteristics of the SS and substrates are summarized in Table 1.
| Item | Type of raw material | ||||
|---|---|---|---|---|---|
| SS | MWS | FW | CoSub | FOG | |
a SS = seed sludge; MWS = municipal waste sludge; FW = food waste; CoSub = co-substrates of municipal waste sludge with food waste at a TS ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; FOG = fat, oil, and grease; VFA = volatile fatty acid. 1; FOG = fat, oil, and grease; VFA = volatile fatty acid. |
|||||
| Density (g mL−1) | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 |
| pH | 7.6 ± 0.1 | 7.4 ± 0.1 | 4.6 ± 0.1 | 6.3 ± 0.1 | 4.3 ± 0.1 |
| sCOD (g L−1) | 0.4 ± 0.5 | 17.9 ± 10 | 103.1 ± 20 | 51.1 ± 20 | 123.0 ± 20 |
| Moisture (%) | 95.4 ± 0.1 | 75.0 ± 0.1 | 60.9 ± 0.1 | 69.5 ± 0.1 | 21.3 ± 0.1 |
| TS (g L−1 substrate) | 40.6 ± 0.2 | 289.9 ± 0.2 | 402.4 ± 0.2 | 333.7 ± 0.2 | 724.5 ± 0.2 |
| VS (g L−1 substrate) | 13.3 ± 0.2 | 113.6 ± 0.2 | 389.7 ± 0.2 | 221.2 ± 0.2 | 718.0 ± 0.2 |
| VS/TS (%) | 31.0 ± 0.2 | 39.0 ± 0.2 | 96.0 ± 0.2 | 61.2 ± 0.2 | 99.0 ± 0.2 |
| VFA (mg L−1) | 617.8 ± 0.5 | 5118.2 ± 0.5 | 2965.0 ± 0.5 | 4278 ± 0.5 | 6371.2 ± 0.5 |
| Alkalinity (mg L−1 as CaCO3) | 1645.8 ± 0.5 | 736.7 ± 0.5 | 2664.6 ± 0.5 | 1488 ± 0.5 | 1136.4 ± 0.5 |
| C/N (w/w) | 5.6 ± 1.0 | 7.4 ± 1.0 | 37 ± 1.0 | 21.9 ± 1.0 | 15 ± 1.0 |
2.2 Reactor set-up and operational strategy
Semi-continuous experiments were performed using three strategies for a total period of 120 days, using single-stage mesophilic continuous stirred-tank reactors (CSTR) with 2.0 L working volume. Three groups of reactors were employed in triplicate as R1(1,2,3), R2(1,2,3) and R3(1,2,3). The 120 day operational period was divided into four periods (30 d per period). The corresponding strategies are shown in Table 2. Each reactor was initially inoculated with 70% seed sludge and 30% co-substrates (MWS + FW). From the next day, feeding was arranged once a day. 100 mL (R1 and R2)/133 mL (R3) of digested materials were withdrawn each day and fed with the same volume to keep a constant HRT of 20 d/15 d. Co-substrates were a mixture of MWS and FW with a TS ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The R1 group received only a mixture of co-substrates based on a percentage of the “safe” organic loading rate (OLR) at 3.0 g VS L−1 d−1 (g volatile solids per reactor volume per day) as a control, according to the optimized results of our preliminary assessment.6 The R2 group received a mixture of co-substrates as well as different FOG contents (4, 6, 8 and 10 mL in 4 periods) as a test. The R3 group received equivalent amounts of co-substrates and FOG contents as R2. However, the HRT of R3 (15 days) was shorter than that of R2 (20 days), to increase the FOG load indirectly via the HRT. The selection of FOG test contents and HRT was based on the ratio of fresh FW ingredients (FW
1. The R1 group received only a mixture of co-substrates based on a percentage of the “safe” organic loading rate (OLR) at 3.0 g VS L−1 d−1 (g volatile solids per reactor volume per day) as a control, according to the optimized results of our preliminary assessment.6 The R2 group received a mixture of co-substrates as well as different FOG contents (4, 6, 8 and 10 mL in 4 periods) as a test. The R3 group received equivalent amounts of co-substrates and FOG contents as R2. However, the HRT of R3 (15 days) was shorter than that of R2 (20 days), to increase the FOG load indirectly via the HRT. The selection of FOG test contents and HRT was based on the ratio of fresh FW ingredients (FW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FOG = 1.7
FOG = 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, m/m) and the fact that most Chinese anaerobic digestion facilities are currently operated at a HRT of 15–18 days. In both R2 and R3, the VS proportions of FOG in the feed were 33%, 42%, 49% and 55% for periods I, II, III, and IV, respectively, as shown in Table 2. All reactors were constantly mixed by magnetic stirrers (RW 205DS1, IKA Works, Inc., USA) at a uniform speed of 200 rpm and the running program was set to 1 h on, then 2 h off. Reactors were immersed and controlled in a water bath within the range of 35 ± 1 °C using bolt electric heating rods. Plastic film was also applied to the top to minimize heat loss. The pH value during this study was not adjusted. Effluent samples were taken every 2 days for the subsequent analysis of anaerobic digestion performance and microbial EPS. Biogas production was measured on a daily basis.
1, m/m) and the fact that most Chinese anaerobic digestion facilities are currently operated at a HRT of 15–18 days. In both R2 and R3, the VS proportions of FOG in the feed were 33%, 42%, 49% and 55% for periods I, II, III, and IV, respectively, as shown in Table 2. All reactors were constantly mixed by magnetic stirrers (RW 205DS1, IKA Works, Inc., USA) at a uniform speed of 200 rpm and the running program was set to 1 h on, then 2 h off. Reactors were immersed and controlled in a water bath within the range of 35 ± 1 °C using bolt electric heating rods. Plastic film was also applied to the top to minimize heat loss. The pH value during this study was not adjusted. Effluent samples were taken every 2 days for the subsequent analysis of anaerobic digestion performance and microbial EPS. Biogas production was measured on a daily basis.
| Period | Group | Substrate (units) | HRT (d) | OLR (g VS L−1 d−1) | FOG% (VS%) | Relative loading (%) |
|---|---|---|---|---|---|---|
| a CoSub = co-substrates of municipal waste sludge with food waste (no FOG contents); FOG = fat, oil, and grease; OLR = organic loading rate. Each reactor was employed in triplicate. | ||||||
| I (1–30 d) | R1(1,2,3) | CoSub | 20 | 3.0 | 0 | 100 |
| R2(1,2,3) | CoSub + FOG (4 mL) | 20 | 4.5 | 33 | 150 | |
| R3(1,2,3) | CoSub + FOG (4 mL) | 15 | 4.5 | 33 | 150 | |
| II (31–60 d) | R1(1,2,3) | CoSub | 20 | 3.0 | 0 | 100 |
| R2(1,2,3) | CoSub + FOG (6 mL) | 20 | 5.2 | 42 | 170 | |
| R3(1,2,3) | CoSub + FOG (6 mL) | 15 | 5.2 | 42 | 170 | |
| III (61–90 d) | R1(1,2,3) | CoSub | 20 | 3.0 | 0 | 100 |
| R2(1,2,3) | CoSub + FOG (8 mL) | 20 | 5.9 | 49 | 200 | |
| R3(1,2,3) | CoSub + FOG (8 mL) | 15 | 5.9 | 49 | 200 | |
| IV (91–120 d) | R1(1,2,3) | CoSub | 20 | 3.0 | 0 | 100 |
| R2(1,2,3) | CoSub + FOG (10 mL) | 20 | 6.7 | 55 | 220 | |
| R3(1,2,3) | CoSub + FOG (10 mL) | 15 | 6.7 | 55 | 220 | |
2.3 Digestion performance analysis
Feed and effluent samples were centrifuged at 8000g for 10 min and then the supernatants were filtered through Millipore disposable filter units (0.45 μm pore size) for the analysis of pH, volatile fatty acids (VFA), alkalinity (Alk), soluble chemical oxygen demand (sCOD) and total nitrogen. pH was determined using a pH meter (Mettler Toledo FE20). VFA, Alk, total solids (TS), volatile solids (VS), density, sCOD and total nitrogen were quantified according to standard methods.20 VFA was measured by titration with H2SO4. VFA = (volume (mL) of H2SO4 from pH 5.0 to pH 4.4 × 1.66) × 500. Alk is represented as mg L−1 CaCO3. All the abovementioned sample analyses were performed in triplicate. Biogas samples from each reactor were obtained daily by an acidified water displacement method under standard conditions (25 °C, 1 atm). The composition of biogas (CH4 and CO2) was determined by a gas chromatograph (GC 2010, Shimadzu) using a thermal conductivity detector equipped with a 2 m × 3 mm stainless-steel packed column (Porapak Q, 80/100 mesh). The oven temperature was maintained at 40 °C during analysis. The injector and detector temperatures were 150 °C and 250 °C, respectively. The results were reported at standard temperature and pressure (STP, 101.325 kPa, 273.15 K). Biogas production was reported as the volume of biogas produced per gram of VSadded (mL per g VSadded).2.4 EPS extraction and analysis
EPS are composed of loosely bound EPS (LB-EPS) and tightly bound EPS (TB-EPS) fractions, based on the extraction methodology. The ingredients and quantities of EPS are strongly dependent on the sample source, the extraction process and the types of analysis conducted.21 In this study, EPS extraction was carried out using a modified heating extraction method that was similar to that of Li et al.22 In brief, a 35 mL digested sample was centrifuged (5810R, Eppendorf) at 8000g for 10 min to remove the supernatant first. Without any delay, the residue was resuspended in PBS solution preheated to 50 °C (0.01 mol L−1, pH = 7.4) to the original volume and vortexed (Vortex-Genie 2, Mo Bio) at 200 rpm for 1 min. The mixture was centrifuged at 8000g for 15 min with the bulk solution and the solid phase collected separately. The organic matter in the bulk solution was collected as LB-EPS. Next, the sludge pellet was rewashed, resuspended to 35 mL in the aforementioned buffer solution and placed in a water bath at 60 °C for 30 min. The sludge mixture was centrifuged again at 8000 g for 15 min with the sediment discarded. The organic matter in the bulk solution was considered as TB-EPS. Both TB-EPS and LB-EPS were extracted in duplicate for each sample. After all the EPS fractions were extracted, 0.45 μm Millipore filter units were used to remove particulates and low-molecular-weight metabolites. LB-EPS and TB-EPS contents were analyzed immediately. In this study, the sum of the amounts of polysaccharides (PS), proteins (PN) and humic acid substances (HS) was used to represent EPS. DNA was used to assess the efficiency and quality of extraction by ranging from 2% to 15% of the total amount of EPS during extraction.23 PS in LB-EPS and TB-EPS were measured by the anthrone method with glucose as the standard.21 PN were measured using a Bradford reagent test kit. HS were measured by a modified Lowry method with humic acid as the standard.24 DNA was analyzed by the diphenylamine reagent colorimetric method using calf thymus DNA as the standard.25 All the abovementioned sample chemical analyses were conducted in triplicate using chemicals of analytical grade. The results of assays are expressed as mean value ± standard deviation.3. Results and discussion
3.1 Process stability
A widely acceptable pH condition, ranging from 6.5 to 7.5, is required for the ACoD process. Especially, methanogens are extremely sensitive to the environmental pH and may become exhausted if they are maintained under this pH limit for a long time, making the digestion system become irreversibly acidified with over-accumulation of VFA. Fig. 1 shows that the pH in R1 remained almost equal to 7.5 during 120 days of operation, whereas this was not the case for R2 and R3. The corresponding values dropped to 6.8 with the addition of FOG during periods I and II, then recovered to the steady-state levels without any artificial assistance for around 60 days. It might be the case that an increase in OLR caused by the daily addition of FOG resulted in slight acidification at the acidogenesis stage. However, owing to the acid–alkaline buffer in the reactors, all systems displayed good acclimation to FOG levels spontaneously, accompanied by the maturation of the microbial community in the digester. Moreover, the fluctuation in pH was associated with a marked variation in the VFA/Alk ratio, which is a reliable indicator of process stability. It is generally recognized that a stable system is achieved when this ratio is less than 0.3–0.4. As can be easily observed from Fig. 1, after a short acclimation (1–10 d) to the mixed substrates, VFA/Alk in R1 decreased to 0.2 gradually after around 40 days, which means that a rather stable digestion system was established without any addition of FOG. Moreover, this ratio practically remained below 0.4 during the entire operation, even at the start-up stage. The result, which revealed that the ACoD system exhibited higher process stability at lower OLR, was also demonstrated by Fernández.26 | ||
| Fig. 1 pH and VFA/Alk ratio in three groups of anaerobic co-digestion reactors during 120 days of operation. | ||
Simultaneously, along with the addition of FOG to R2 and R3, a substantial increase in the VFA/Alk ratio was observed during period I. When the FOG content increased to 42% (on the basis of VS), this ratio rapidly rose to peak values of 0.47 and 0.56 for R2 and R3, respectively, on day 31. These values, which are close to the threshold, indicate that low system stability and an unfavorable balance between acidogenic and methanogenic microorganisms emerged, resulting in acidification of the digester. Nutritional balance with the addition of FOG depends on faster hydrolysis and acidogenesis steps, which might generate large amounts of VFA and a drop in pH. Excessive VFA production can reportedly inhibit the digestion process.27 However, the accumulated VFA were gradually utilized by predominant methanogens in the following days. The ratio was well above the limit and decreased to 0.2 without artificial assistance until day 60. Typically, this ratio in R3 was slightly higher than in R2 during days 1 to 60. One reason might be that the shorter HRT in R3 induced more washout of active methanogens during effluent removal and then OLR increased indirectly when an equal load was received.
Fluctuations in the pH and VFA/Alk ratio revealed that FOG might disturb the system stability, and a longer accommodation period was required (60 days in R2 and R3, compared with 40 days in R1). During periods III and IV (FOG VS% of 49% and 55%), no major variations occurred in pH and VFA/Alk, which may be explained by the fact that the FOG load in this study was acceptable and the potential for higher loading could be investigated.
3.2 General performance
Based on the experimental results from Table 3, the trend in VS concentrations was very similar to that of sCOD. VS and sCOD contents exhibited minor changes in the R1 control digester when this received MWS + FW only. VS and sCOD concentrations in R2 and R3 reached peaks when the FOG content reached 42% (VS%). However, with a 60 day adaptation period, VS and sCOD levels in R2 and R3 dropped to those in R1 in periods III and IV, accompanied by optimal operation of the digester. The data prove that the readily decayed solid organic materials were rapidly degraded by the microorganisms, as it can also be demonstrated by the elevated VS/TS ratio. The VS and sCOD concentrations in R3 were consistently lower than those in R2. By taking into account the fact that the two experimental systems operated at the same OLR, it is suggested that the shorter HRT in R3 resulted in the outflow of much more VS in the effluent.| Parameter | Period I | Period II | Period III | Period IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R1 | R2 | R3 | R1 | R2 | R3 | R1 | R2 | R3 | |
| VS (g L−1) | 15.6 ± 0.7 | 20.1 ± 0.7 | 17.4 ± 0.3 | 16.1 ± 0.3 | 21.2 ± 0.7 | 19.6 ± 0.4 | 16.4 ± 0.3 | 18.8 ± 0.3 | 16.7 ± 0.2 | 15.6 ± 0.2 | 16.9 ± 0.2 | 14.9 ± 0.2 |
| VS/TS (%) | 36.9 ± 1.4 | 46.3 ± 1.5 | 44.3 ± 1.4 | 43.2 ± 1.9 | 53.0 ± 1.7 | 54.8 ± 1.6 | 48.8 ± 1.1 | 55.1 ± 1.9 | 51.8 ± 1.3 | 53.5 ± 1.3 | 54.0 ± 2.0 | 55.9 ± 1.8 |
| sCOD (mg L−1) | 440.2 ± 13.2 | 1257.6 ± 31.5 | 1148.0 ± 27.7 | 553.8 ± 11.5 | 1533.5 ± 31.2 | 1302.1 ± 20.8 | 550.2 ± 11.9 | 725.6 ± 12.0 | 718.1 ± 9.3 | 552.2 ± 9.7 | 699.4 ± 8.4 | 689.8 ± 9.6 |
| sCOD reduction (%) | 61.8 ± 1.7 | 67.7 ± 1.9 | 63.8 ± 2.2 | 60.2 ± 2.4 | 69.6 ± 1.8 | 64.4 ± 2.0 | 61.0 ± 1.3 | 74.2 ± 1.9 | 70.9 ± 1.9 | 61.5 ± 1.7 | 77.1 ± 2.1 | 75.7 ± 2.2 |
| Biogas yield (mL d−1) | 1504 ± 20.8 | 2698 ± 33.6 | 2552 ± 37.5 | 1672 ± 21.0 | 4181 ± 34.6 | 3493 ± 29.3 | 1690 ± 17.6 | 3604 ± 31.7 | 3550 ± 29.6 | 1618 ± 16.8 | 2219 ± 18.3 | 2123 ± 19.7 |
| Biogas yield (mL per g VSadded) | 501.3 ± 6.9 | 611.3 ± 7.5 | 578.5 ± 8.4 | 557.5 ± 7.1 | 810.3 ± 6.7 | 677.2 ± 5.7 | 563.6 ± 5.9 | 613.0 ± 5.4 | 603.8 ± 5.0 | 539.3 ± 5.6 | 336.2 ± 2.8 | 321.8 ± 2.9 |
| Biogas promotion (%) | — | 21.9 ± 7.1 | 15.4 ± 7.3 | — | 45.3 ± 6.8 | 21.5 ± 5.9 | — | 8.8 ± 5.9 | 7.1 ± 5.3 | — | −37.7 ± 4.7 | −40.3 ± 4.9 |
Furthermore, the final percentages of sCOD removal in R2 and R3 reached 77% and 75%, respectively, compared with 61% in R1. Two FOG-enhanced digestions resulted in dramatically higher sCOD removal rates, which were consistent with other studies reported that a high content of organic materials has a positive effect on co-digestion processes.28 These findings proved that co-digestion with FOG and a longer HRT have more advantages in the conversion of organics, which are probably due to the balanced nutrient ratio with the mixed substrates and an enhanced pH buffering capacity.
3.3 Biogas production
Employing MWS as the sole substrate has limited the successful implementation of biogas production, owing to the low C/N ratio contained in sewage sludge. This ratio, which is of the order of 6–16 (w/w), is regarded as a serious problem in anaerobic digestion. It should range from 20 to 30 to ensure a sufficient supply of nitrogen for cell production and degradation of the carbon present in the process. Fig. 2(a) presents the changes in the daily production of biogas (per g VSadded) in the three digestion units during four periods. The average biogas production in R1 was equal to 540 mL per g VSadded as a control, and the corresponding C/N ratio in the co-substrate reached 21.9. In comparison, biogas production in the two test units apparently fluctuated when these received mixtures with elevated FOG contents. Along with an increase in FOG content from 33% to 42% (VS%), peaks in biogas yield (about 862 and 715 mL per g VSadded) were achieved for R2 and R3 around day 44 and day 40, respectively. More specifically, co-digestion with FOG content at 42% (VS%) and OLR at 5.2 g VS L−1 d−1 with HRT up to 20 days resulted in an increase in biogas yield by 45%, which demonstrates that a delicate balance was achieved between the rates of hydrolysis/acidogenesis and methanogenesis. However, a remarkable decrease in biogas production was observed in the two test units around day 60. Subsequently, a reduction below the level in R1 was recorded at day 80. The further increase in FOG content exerted a negative effect on the biogas yield. Biogas production was significantly inhibited by 37.7% and 40.3% for R2 and R3, respectively, during period IV with a progressive increase in FOG content to 55% (VS%): only 336.2 and 321.8 mL per g VSadded were achieved. Therefore, a FOG content of 42% (VS%) and an OLR of 5.2 g VS L−1 d−1 was found to be optimum for maximum waste treatment capacity while still maximizing the yield of biogas from the process. A greater biogas output in a FOG-enhanced process was also reported by Davidsson and Luostarinen.9,29 However, according to Luostarinen et al.,29 at an upper limit of FOG content (of the order of 55% on the basis of VS) degradation was incomplete and biogas yield decreased. Martinez et al. also found that treating a mixture with a higher content of lipid-rich waste resulted in a decrease in the specific production of methane, although an adaptation period was employed with the reactor.30 Another reason why a lower gas yield was obtained was the adsorption of FOG components onto sludge, which then would have precluded degradation by microorganisms.31 | ||
| Fig. 2 Daily biogas production per VSadded (a) and cumulative biogas production (b) in three groups of anaerobic co-digestion reactors during 120 days of operation. | ||
Moreover, Fig. 2(b) shows that the cumulative biogas yield in R1 during 120 days of the process was about 195 L. The levels in R2 and R3 were much higher than in R1: 381 L and 351 L were obtained, respectively. The promotion ratios of 95% (381 vs. 195 L) and 80% (351 vs. 195 L) were similar to the mean value of the relative OLR (85%) over the four periods, which proves that the organic loading that was present in FOG significantly contributed to biogas production. In addition, the more efficient conversion of organic material in R2 (95%) than in R3 (80%) was also in accordance with the aforementioned performance analysis. It is likely that the increase in HRT would drive the performance improvements.
Specifically, the fact that biogas production (per g VSadded) was higher in R3 than in R2 by about 11.6% (average 547 vs. 490 mL per g VSadded) was noted during the initial feeding stage (1–14 d). It is possible that in R3 the shorter HRT increased the OLR indirectly, which resulted in more biogas being harvested temporarily.
Martinez et al. also reported an increase in biogas production with a decrease in HRT (increase in organic loading rate). This behavior may be rationalized by the low complexity of the substrate, which enabled its rapid conversion.31
Additionally, R2 increased in daily production from day 14 and the cumulative biogas yield increased from day 24, as the black circles indicate in Fig. 2. It was anticipated that a vigorous digestion system may become prematurely exhausted and induce a reduction in biogas production, which was considered as “doping”.
Furthermore, changes in the biogas composition in the three reactors were characterized by GC. As revealed in Fig. 3, it is clear that CH4 was dominant in biogas after co-digestion, especially for the systems with added FOG, with increases to average contents of 70.9% and 66.4% for R2 and R3, respectively. It was concluded that the adaptation of biomass to FOG content was a rather gradual process. The increase in the efficiency of biogas conversion confirmed that FOG had positive effects on the hydrolysis rate and methane potential, which were attributed to well-functioning methanogens that scavenged the organic acids formed by acidogenic bacteria.32
 | ||
| Fig. 3 Changes in biogas composition after anaerobic co-digestion with different operational strategies. | ||
3.4 Variation in extracellular polymeric substances (EPS)
 | ||
| Fig. 4 Cumulative EPS concentrations and proportions of LB-EPS and TB-EPS in three groups of anaerobic co-digestion reactors during 120 days of operation. | ||
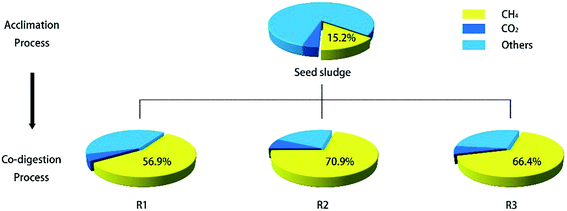
At the beginning (1–20 d) of feeding with different digestion substrates, a large decrease in total EPS was observed. The decrease in EPS fractions in the three reactors is probably attributed to the fact that the microorganisms underwent a sudden stepwise increase in organic loading, overproduced hydrogen due to this change and subsequently readjusted their operation based on the newly employed conditions, slowing down their metabolic operations. At the end of the accommodation stage (20–30 d), each system arrived at a vigorous phase of EPS accumulation gradually from day 20 to day 70, due to major stimulation of the active digesting microbial population as a result of loading. These results seem to confirm that microorganisms were progressively acclimated to the new co-substrates, which were demonstrated in the abovementioned performance analysis.
Specifically, by adding FOG to the two test units, a more significant accumulation of EPS was obtained. R2 and R3 reached a plateau stage for EPS earlier and stayed longer (around days 30–60) compared with R1 (day 65 only). For the whole 120 days of operation, the total EPS concentration in the test units was found to increase by 19.4% and 5.2% for R2 and R3, respectively, in this study, averaging 503.6 and 443.9 vs. 422.0 mg L−1 for R1. The EPS concentration peaked at day 39 (772.3 mg L−1), day 55 (640.9 mg L−1) and day 67 (648.5 mg L−1) for R2, R3 and R1 successively. In fact, anaerobic digestion with high loading is liable to cause the accumulation of soluble microbial products.33 This is because there are abundant microbial residues in the organic waste digestate following digestion, which leads to the possibility of microbial EPS accumulation.34 Based on the operational mode of the CSTR, equal volumes of digestion material were withdrawn and fed to maintain a constant working volume of 2.0 L in each reactor. The effluent samples collected were mutually independent. In this study, substrate degradation and microbe release are regarded as the two major sources of EPS content. In fact, variations in EPS content from the point of view of the substrate were controlled identically by adding equivalent amounts of co-substrate (MWS + FW at 3.0 g VS L−1 d−1) to all reactors. Therefore, fluctuations in EPS contents can be attributed to cell lysis in the active microbial population in response to FOG. The release of intracellular organics benefited microbial reproduction and produced more EPS in FOG-enhanced systems. Ng et al.35 also reported that biomass underwent endogenous respiration and cell lysis would occur at longer mean cell residence times, which would cause the further release of EPS into the bulk solution. Moreover, R2 produced more EPS at a longer HRT than R3. Many researchers have found that EPS in various microbial aggregates increase with an increase in HRT.22 In addition, the sCOD loading also has a significant effect on EPS accumulation.33 The relatively longer HRT in R2 and higher sCOD loading in R2 and R3 (1300–1500 mg L−1) might generate more EPS than at the lower sCOD loading in R1 (550 mg L−1). Therefore, the specific increases in EPS in R2 and R3 were reasonable.
It is interesting to note that unlike conditions of stable biogas yield, a decrease in EPS in R1 from day 60 was observed without interference from FOG. EPS levels in the FOG-enhanced units of R2 and R3 declined even further below in the control unit at around day 70. It could be inferred that a digestion unit running for a long period (over 60 days) might produce large amounts of toxic metabolic products that prevent the bacterium from generating EPS. Enhanced digestion systems may become exhausted prematurely due to the accumulation of products of metabolism, which was described as a “doping” phenomenon before.
Nevertheless, the direct toxicity of excessive FOG to the digesting microbial community and changes in microbial metabolism should also be taken into consideration. In fact, high FOG contents of organic wastes can lead to the accumulation of inhibitory compounds such as long-chain fatty acids (LCFAs). It is well known that the accumulation of LCFAs may inhibit anaerobic digestion because of their direct toxicity toward acetogens and methanogens, which are the two main groups involved in the breakdown of LCFAs.36 Another inhibitory mechanism is the adsorption of surface-active acids onto the cell wall,37 which affects the processes of transportation and protection. Sutherland et al.38 pointed out that aggregations of microorganisms can provide EPS as an energy source and protective layer for cells against damage by toxic substances from a harsh external environment. With an increase in FOG content, the products of EPS were able to combine with LCFAs, being greatly stimulated due to the protective response of the microorganism.
Fig. 4 shows that the proportion of LB-EPS in microbial aggregates was always less than that of TB-EPS, accounting for about 40% (LB-EPS) and 60% (TB-EPS) in the three reactors during 120 days of operation. Such a discrepancy may be attributed to harsh conditions in the extraction procedure of TB-EPS, which involved heating in a 60 °C water bath. Another reason might be that the loose binding that existed in LB-EPS led to ready fluctuation during the digestion process. LB-EPS in sludge flocs would act as the primary surface for cell attachment and sludge flocculation. In a recent study, the LB-EPS content was found to be more closely related to microbial activity, whereas no obvious correlation could be found between the TB-EPS concentration and microbial aggregates.22
 | ||
| Fig. 5 Heat map of EPS subfractions in three groups of anaerobic co-digestion reactors during 120 days of operation. | ||
Among all three reactors, R2 and R3 yielded much higher EPS concentrations than R1 for both humic acid substances (HS) and proteins (PN). In addition, HS were the predominant components with corresponding concentrations of 194, 254 and 221 mg L−1, followed by PN at 110, 141 and 130 mg L−1 for R1, R2 and R3, respectively. PS were dispersed evenly, accounting for a small but stable proportion of EPS amounts at 20–30% (see ESI†). It was noted that in the subfractions similar cumulative peaks in HS occurred in the three reactors, which were in accordance with the total variation in EPS as revealed in Fig. 4. These findings also indicate that the relative contribution of cumulative EPS was mainly attributed to HS components. One concern is that there are many humic-like substances produced from the degradation of feed substrates. In studying primary sludge digestion, Miron et al.41 reported that the hydrolysis of lipids and carbohydrates increased with an increase in solid retention time, whereas protein hydrolysis only occurred under methanogenic conditions.
According to previous reports, EPS play an important role in microbial adhesion and aggregation processes, promoting the formation and stability of the microbial community structure.16,42 Each EPS fraction contains different components and exhibits rather distinct chemical properties. The polysaccharides and proteins in TB-EPS are independent of the influent carbon source and C/N ratio.43,44 However, the protein content and carbohydrate content in LB-EPS are related to the influent C/N ratio.44 Such differences indicate that the different EPS fractions had different components.45
The p-value was determined as follows:
Then, the indicator k was given by the absolute value of p. The k-value was expressed as follows:
A higher k-value represents an increased degree of fluctuation in EPS. As can be observed in Fig. 6, the disparities in the k-values of PS and HS in TB-EPS were less significant than those in LB-EPS, as the black and grey ovals indicate. Moreover, these findings also suggest that the degree of change in LB-EPS subfractions appears to be more obvious than that in TB-EPS. Although the metabolism was considered to be capable of dissolving bound EPS in the supernatant, in the meantime, TB-EPS released from the inner cells experienced difficulty in diffusing out of the sludge. The variation in LB-EPS that was observed in this study in the presence of FOG was expected to be related more directly to different levels of microbial EPS secretion as active responses to external environmental challenges.
 | ||
| Fig. 6 Degree of change in LB-EPS and TB-EPS subfractions in three groups of anaerobic co-digestion systems, which was described by the k-value. | ||
Furthermore, compared to the variations in EPS subfractions with changes in the process conditions, the extent of the changes in PN was the most remarkable. The k-values of PN were dramatically higher than those of other subfractions and there was a trend of change that was correlated to the operational conditions, as the red arrows indicate. PN are believed to play a crucial role in the structure, properties and functions of sludge aggregates.46 The variation in the PN concentration might be attributed to the presence of a large quantity of exoenzymes, as suggested by Frølund et al.24 The easy degradation and uptake of readily biodegradable organic substrates, such as glucose and acetate, gives rise to a high level of exoenzymes in the EPS matrix.47 The higher k-value of PN compared with that of any subfractions proved that the substrates that were obtained from the digested materials were readily biodegradable.
4. Conclusions
Mesophilic co-digestion of MWS with FW at suitable FOG contents led to substrates that were better balanced and efficiently degradable. Biogas production and COD reduction were increased significantly in the FOG test systems. However, excessive addition of FOG disturbed the stability of the process and restricted digestion performance. Variations in EPS revealed that the microbial activity was affected by FOG. Each EPS subfraction plays a different role in microbial metabolic activities due to its distinct chemical properties. Analysis of EPS also indicated that FOG-enhanced systems may become exhausted prematurely due to “doping” phenomena. In general, the complexity and extent of synergistic interactions in the microbial world during ACoD is largely unexplored and further study remains an essential step towards optimizing digestion performance.Acknowledgements
This study was sponsored by the National Natural Science Foundation of China (Grants No. 51378189, 51578223 and 51521006). Thanks to Li Zhou for aid in polishing the language.References
- M. T. Nguyen, N. H. M. Yasin, T. Miyazaki and T. Maeda, Chemosphere, 2014, 117, 552–558 CrossRef CAS PubMed.
- N. B. o. S. o. China, China statistical yearbook, China statistics press, Beijing, 2014 Search PubMed.
- J. H. Long, T. N. Aziz, F. L. D. L. Reyes Iii and J. J. Ducoste, Process Saf. Environ. Prot., 2012, 90, 231–245 CrossRef CAS.
- Q. Wang, L. Ye, G. Jiang, P. D. Jensen, D. J. Batstone and Z. Yuan, Environ. Sci. Technol., 2013, 47, 11897–11904 CrossRef CAS PubMed.
- L. Björnsson, M. Murto and B. Mattiasson, Appl. Microbiol. Biotechnol., 2000, 54, 844–849 CrossRef.
- C. Gou, Z. Yang, J. Huang, H. Wang, H. Xu and L. Wang, Chemosphere, 2014, 105, 146–151 CrossRef CAS PubMed.
- P. G. Stroot, K. D. McMahon, R. I. Mackie and L. Raskin, Water Res., 2001, 35, 1804–1816 CrossRef CAS PubMed.
- K. Fricke, H. Santen, R. Wallmann, A. Hüttner and N. Dichtl, Waste Manage., 2007, 27, 30–43 CrossRef CAS PubMed.
- Å. Davidsson, C. Lövstedt, J. la Cour Jansen, C. Gruvberger and H. Aspegren, Waste Manage., 2008, 28, 986–992 CrossRef PubMed.
- C. Li, P. Champagne and B. C. Anderson, Bioresour. Technol., 2011, 102, 9471–9480 CrossRef CAS PubMed.
- C. Noutsopoulos, D. Mamais, K. Antoniou, C. Avramides, P. Oikonomopoulos and I. Fountoulakis, Bioresour. Technol., 2013, 131, 452–459 CrossRef CAS PubMed.
- J. Moestedt, E. Nordell, Y. S. Shakeri, J. Lundgren, M. Martí, C. Sundberg, J. Ejlertsson, B. H. Svensson and A. Björn, Waste Manage., 2015 DOI:10.1016/j.wasman.2015.03.007.
- M. T. Madigan, J. M. Martinko and T. D. Brock, Brock Biology of Microorganisms, 2006, pp. 294–295 Search PubMed.
- N. Ganidi, S. Tyrrel and E. Cartmell, Bioresour. Technol., 2009, 100, 5546–5554 CrossRef CAS PubMed.
- B. Demirel and P. Scherer, Rev. Environ. Sci. Biotechnol., 2008, 7, 173–190 CrossRef CAS.
- G. Sheng, H. Yu and X. Li, Biotechnol. Adv., 2010, 28, 882–894 CrossRef CAS PubMed.
- S. Comte, G. Guibaud and M. Baudu, Enzyme Microb. Technol., 2006, 38, 237–245 CrossRef CAS.
- Z. Yu, X. Wen, M. Xu and X. Huang, Bioresour. Technol., 2012, 117, 333–340 CrossRef CAS PubMed.
- G. F. Bennett, J. Hazard. Mater., 2004, 106, 177 Search PubMed.
- APHA, Standard Methods for the Examination of Water and Wasterwater, American Public Health Association, Washington, DC, 21st edn, 2005 Search PubMed.
- B. Frølund, R. Palmgren, K. Keiding and P. H. Nielsen, Water Res., 1996, 30, 1749–1758 CrossRef.
- X. Y. Li and S. F. Yang, Water Res., 2007, 41, 1022–1030 CrossRef CAS PubMed.
- B. Q. Liao, D. G. Allen, I. G. Droppo, G. G. Leppard and S. N. Liss, Water Res., 2001, 35, 339–350 CrossRef CAS PubMed.
- B. Frølund, T. Griebe and P. H. Nielsen, Appl. Microbiol. Biotechnol., 1995, 43, 755–761 CrossRef.
- Y. Liu and H. H. P. Fang, Crit. Rev. Environ. Sci. Technol., 2003, 33, 237–273 CrossRef CAS.
- A. Fernández, A. Sánchez and X. Font, Biochem. Eng. J., 2005, 26, 22–28 CrossRef.
- A. Nordberg, A. Jarvis, B. Stenberg, B. Mathisen and B. H. Svensson, Bioresour. Technol., 2007, 98, 104–111 CrossRef CAS PubMed.
- H. M. El-Mashad and R. Zhang, Bioresour. Technol., 2010, 101, 4021–4028 CrossRef CAS PubMed.
- S. Luostarinen, S. Luste and M. Sillanpää, Bioresour. Technol., 2009, 100, 79–85 CrossRef CAS PubMed.
- E. J. Martinez, V. Redondas, J. Fierro, X. Gómez and A. Morán, J. Residuals Sci. Technol., 2011, 8, 53–60 CAS.
- E. J. Martínez, J. Fierro, M. E. Sánchez and X. Gómez, Int. Biodeterior. Biodegrad., 2012, 75, 1–6 CrossRef.
- M. Ros, I. H. Franke-Whittle, A. B. Morales, H. Insam, M. Ayuso and J. A. Pascual, Bioresour. Technol., 2013, 136, 1–7 CrossRef CAS PubMed.
- F. Lü, L. Hao, D. Guan, Y. Qi, L. Shao and P. He, Water Res., 2013, 47, 2297–2306 CrossRef PubMed.
- F. Lü, Q. Zhou, D. Wu, T. Wang, L. Shao and P. He, Chem. Eng. J., 2015, 262, 932–938 CrossRef.
- H. Y. Ng, T. W. Tan and S. L. Ong, Environ. Sci. Technol., 2006, 40, 2706–2713 CrossRef CAS PubMed.
- K. Hanaki, T. Matsuo and M. Nagase, Biotechnol. Bioeng., 1981, 23, 1591–1610 CrossRef CAS.
- M. M. Alves, J. A. Vieira, R. M. Pereira, M. A. Pereira and M. Mota, Water Res., 2001, 35, 264–270 CrossRef CAS PubMed.
- I. Sutherland, Microbiology, 2001, 147, 3–9 CrossRef CAS PubMed.
- T. L. Poxon and J. L. Darby, Water Res., 1997, 31, 749–758 CrossRef CAS.
- G. Long, P. Zhu, Y. Shen and M. Tong, Environ. Sci. Technol., 2009, 43, 2308–2314 CrossRef CAS PubMed.
- Y. Miron, G. Zeeman, J. B. van Lier and G. Lettinga, Water Res., 2000, 34, 1705–1713 CrossRef CAS.
- H. C. Flemming and J. Wingender, Nat. Rev. Microbiol., 2010, 8, 623–633 CAS.
- F. Ye, G. Peng and Y. Li, Chemosphere, 2011, 84, 1250–1255 CrossRef CAS PubMed.
- F. Ye, Y. Ye and Y. Li, J. Hazard. Mater., 2011, 188, 37–43 CrossRef CAS PubMed.
- C. S. Zhang Zhaoji, W. Shumei and L. Hongyuan, Appl. Microbiol. Biotechnol., 2011, 89, 1563–1571 CrossRef PubMed.
- P. le-Clech, V. Chen and T. A. G. Fane, J. Membr. Sci., 2006, 284, 17–53 CrossRef CAS.
- D. T. Sponza, Enzyme Microb. Technol., 2003, 32, 375–385 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21459a |
| This journal is © The Royal Society of Chemistry 2015 |