Unique lamellar lyotropic liquid crystal phases of nonionic phytosterol ethoxylates in glycerol†
Zhaohong Qiana,
Xiu Yueb,
Sijing Yia,
Qintang Lia and
Xiao Chen*a
aKey Laboratory of Colloid and Interface Chemistry, Shandong University, Ministry of Education, Jinan, 250100, China. E-mail: xchen@sdu.edu.cn; Fax: +86-531-88564464; Tel: +86-531-88365420
bLaboratory of Environmental Sciences and Technology, Xinjiang Technical Institute of Physics & Chemistry, Key Laboratory of Functional Materials and Devices for Special Environments, Chinese Academy of Sciences, Urumqi, 830011, China
First published on 20th November 2015
Abstract
As one type of biocompatible surfactant, phytosterol ethoxylates (BPS-n, n is the oxyethylene chain length) have attracted more and more attention for their characteristic molecular structure. In this paper, the aggregation behaviors of BPS-5 or BPS-10 with five or ten oxyethylene units were investigated in glycerol to explore the solvent molecular structure effect, especially at higher surfactant concentration regions. Surface tension measurements were adopted to analyze the thermodynamic process for micelle formation. Polarized optical microscopy and small-angle X-ray scattering were then used to identify and characterize the formed lyotropic lamellar liquid crystalline (LLC) phase structures. An interesting coexistence of two kinds of lamellar phases in the BPS-5/glycerol system was found, which was attributed to different hydrogen bonding interactions between BPS-5 and glycerol. More intermolecular interaction information was mapped through the rheological measurement and Fourier transformed infrared (FTIR) spectroscopy. The relatively high self-assembling capability of glycerol was recognized to result from its molecular structure with more hydroxyl groups. Its higher Gordon parameter benefitting from the stronger hydrogen-bonded networks made the glycerol exhibit obvious enhancement on the LLC phase formation of BPS-5 or BPS-10, compared to those in water or amide solvents (wider concentration range of LLC phase and higher viscoelasticity). Such a stronger organized solvent structure in glycerol might originate from the special three-dimensional hydrogen-bonding patterns and much higher lifetimes compared to those for water.
1. Introduction
Sterol ethoxylate surfactants have attracted much attention due to their good biocompatibility, low toxicity, and many applications in the fields of detergents, cosmetics, medicine and food industry, etc.1 Because of the rigid sterol ring structures, they usually exhibit a much stronger segregation tendency between the hydrophilic and hydrophobic parts compared to the conventional alkyl ethoxylated nonionic surfactants. The aggregates formed in solutions could therefore exhibit certain characteristics of “rigidity”. Various aggregate morphologies including micelles,2–4 vesicles,5–8 and liquid crystals9–16 have been observed in different solvents. In aqueous systems, Abe et al. have carried out systematic investigations on phase behaviors of phytosterol ethoxylate surfactants (BPS-n, n is an oxyethylene chain length).17–21 They disclosed a network structure of micelles formed by polyoxyethylene cholesteryl ether and lauryl diethanolamide.18 By adding monoglyceride (monolaurin or monopalmitin) in aqueous solution, however, the temperature-responsive wormlike micelles could be obtained.20 Folmer et al. have also studied the effect of polyoxyethylene chain length on BPS-n aggregation properties22 and found the decreased critical micelle concentration (CMC) values in water with the reduction of the hydrophilic oxyethylene chain length.23 In aqueous solution of a short chain polyoxyethylene cholesterol ether (ChEO3), Kunieda et al. observed the formation of a lamellar phase (Lα) when the concentration was above 75%, which could be re-dispersed into vesicles in excess water.24 Richer morphologies were then mapped in BPS-15/H2O system with the successively formation of micellar, micellar cubic, hexagonal, lamellar, and solid phases with increasing concentration of BPS-15.25To broaden the solvent media for BPS-n self-assembling and explore more potential properties of their aggregates, researchers have also paid their attention to non-aqueous systems of BPS-n. In room temperature ionic liquid (RTIL) of 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim]PF6), Abe et al. studied the phase behavior of BPS-n with different PEO chains.26 Compared to the BPS-n/H2O system, the notable difference in phase types was found and higher BPS-n concentrations were required to induce phase transitions. We have also compared the aggregation behaviors of BPS-10 in two ionic liquids, a protic ethylammonium nitrate (EAN) and an aprotic 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4).27,28 The lyotropic liquid crystal (LLC) phase regions in EAN were found to appear at lower surfactant concentrations than those in [Bmim]BF4. Instead of only one Lα phase formation in [Bmim]BF4, an extra hexagonal phase (H1) was observed in EAN due to its higher Gordon parameter and stronger hydrogen-bonded network. Therefore, the protic solvent behaves more effective than the aprotic one as the self-assembling media. This point has been verified in other polar organic solvents with water-like structures, like glycerol, formamide, and ethylene glycol.29–32 In our study on BPS-10 phase behaviors using three organic amide compounds (FA, NMF and DMF) as solvents, the H1 and Lα phases could also be obtained due to the existence of similar hydrogen-bonded networks.33
As a continuation of our research in this paper, we extended the study of BPS-n aggregation behavior to a new solvent medium, the glycerol. This is not only because of its excellent solvent characteristics of a high cohesive energy or dielectric constant, but also due to its potential applications in pharmaceutical and cosmetic products, where it is usually used as co-solvent to improve or modulate the surfactant aggregations.34–40 For examples, in the study of self-assembly of alkyl-propoxy-ethoxylate surfactants, Alexandridis et al. found the increased surfactant solubility with introducing such alcohol cosolvents into water and thus an increased CMC.34 The liquid crystalline phases (hexagonal and lamellar), isotropic and gel-like phases could also be obtained in glycerol-containing solvent system as reported by Dorfler et al.37 for long-chain carboxylic acid potassium (K-soap) and by Auvray et al. for ionic surfactants in polar solvents.38,39 Usually, only the lamellar phase was formed in systems with glycerol or ethylene glycol as solvents. Therefore, it is expected to explore the phase behaviors of BPS-n surfactants in such alcohol solvents. The capabilities of adjusting such low toxic and environmentally friendly surfactant interactions using these non-aqueous polar solvents should practically lead to extension of their applications in the pharmaceuticals and cosmetics formulations.
2. Experimental
2.1. Materials
The nonionic ethoxylated phytosterol surfactants BPS-n (n = 5 and 10) with the chemical structures shown in Fig. 1, were supplied as kindly gifts by Nikko Chemicals from Japan. The hydrophobic portion of the BPS-n surfactants was consisted of β-sitosterol, campesterol, and stigmasterol in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and less than 3 wt% polyoxyethylene was contained as a hydrophilic impurity. Glycerol (A.R. grade) was purchased from Sinopharm Chemicals Reagent Co. Both the surfactants and glycerol were used without further purification.
1 and less than 3 wt% polyoxyethylene was contained as a hydrophilic impurity. Glycerol (A.R. grade) was purchased from Sinopharm Chemicals Reagent Co. Both the surfactants and glycerol were used without further purification.
2.2. Sample preparation
All samples were prepared by mixing the BPS-n surfactant and glycerol with exact mass ratios. The mixtures were heated to about 80 °C and stirred until the samples became completely transparent without any un-dissolved ingredients. After several cycles of heating, vortex-mixing and centrifugation, the samples were fully mixed and then equilibrated at 25 °C for more than three weeks.2.3. Characterization
3. Results and discussion
3.1. Micellization behavior of BPS-n in glycerol
As a polar organic solvent, the glycerol exhibited good self-assembling capability, which could be certified by the CMC value for a surfactant determined by the surface tension (γ) measurement. The measured γ profiles with concentrations (weight percentage wt%) for both BPS-5 and BPS-10 in glycerol were shown in Fig. 2. At low concentrations, γ was decreased gradually with increasing concentration until a plateau was reached with a sharp break point, indicating the interfacial saturation of surfactant and the initialization of micelle formation. The CMC values were assigned from the break points with the results of about 4.0 × 10−3 wt% (7.5 × 10−5 mol L−1) and 5.6 × 10−3 wt% (9.2 × 10−5 mol L−1) respectively for BPS-5 and BPS-10. Both CMC values in glycerol were larger than that of BPS-10 in water (∼1.0 × 10−5 mol L−1),23 but lower than those in EAN or [Bmim]BF4 (∼3 × 10−4 mol L−1),28 reflecting the better capability of glycerol as self-assembling medium than ionic liquids. Meanwhile, the similar CMC values of BPS-5 and BPS-10 indicated that the solvophobic interaction should dominate the aggregate formation because they were of the same solvophobic parts. The a little larger CMC of BPS-10 than BPS-5 was perhaps due to its longer solvophilic EO chains.From the surface tension measurements, several other thermodynamic parameters, including the effectiveness of the surface tension reduction (ΠCMC), the surface excess at the air/glycerol interface (Γmax) and the minimum occupied area per surfactant molecule adsorbed at the interface (Amin), were also obtained based on the following equations:
| ΠCMC = γ0 − γCMC | (1) |
 | (2) |
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C is the slope of γ vs. ln
C is the slope of γ vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C profile when the concentration is near CMC. NA is Avogadro's number, and R (= 8.314 J mol−1 K−1) is the gas constant. The calculated data were all listed in Table 1. Little difference between BPS-5 and BPS-10 were found for values of γCMC and ΠCMC, reflecting their similar surface activities. The increased Γmax and decreased Amin values of BPS-10, however, suggested an enhanced solvophilic interaction with longer EO chains. In addition, this Amin of BPS-10 in glycerol was even smaller than that in EAN,28 indicating a more dense packing of molecules between glycerol solvent and EO chains, which should be therefore more extended in glycerol.
C profile when the concentration is near CMC. NA is Avogadro's number, and R (= 8.314 J mol−1 K−1) is the gas constant. The calculated data were all listed in Table 1. Little difference between BPS-5 and BPS-10 were found for values of γCMC and ΠCMC, reflecting their similar surface activities. The increased Γmax and decreased Amin values of BPS-10, however, suggested an enhanced solvophilic interaction with longer EO chains. In addition, this Amin of BPS-10 in glycerol was even smaller than that in EAN,28 indicating a more dense packing of molecules between glycerol solvent and EO chains, which should be therefore more extended in glycerol.
| CMC (wt%) | γCMC (mN m−1) | ΠCMC (mN m−1) | Γmax (μmol m−2) | Amin (Å2) | |
|---|---|---|---|---|---|
| BPS-5 | 4.0 × 10−3 | 23.2 | 40.1 | 7.19 | 23.1 |
| BPS-10 | 5.6 × 10−3 | 24.6 | 38.7 | 10.08 | 16.47 |
The behavior of glycerol as a solvent here was consistent with its self-assembling capability as indicated by Gordon parameter, which could be used to measure the cohesive energy density of solvent. Generally, the higher the G values, the stronger the driving force for the self-assembly process. For water, glycerol, EAN and [Bmim]BF4, the Gordon parameter values are respectively 2.8, 1.51, 1.4 and 0.79 J m−3.41 The existed data difference reflects the ordering difference between these solvent bulk structures. Considering the protic nature of these solvents, it was also an indication for the ordering of the hydrogen bonding network formed inside the bulk solvent. At higher surfactant concentrations, however, more complicated intermolecular actions might reduce such differences as reflected by following results.
3.2. LLC formation in BPS-n/glycerol systems
The POM and SAXS measurements were then employed to identify the phase structures of the samples at higher concentrations (CBPS-5 > 50%). From the POM photographs shown in Fig. 4, the “oily streaks” textures could be observed. The upper anisotropic one in two-phase separated samples at CBPS-5 = 25% and 40% were also shown in Fig. S1† with the “Maltese cross” and “oily streaks” textures, which usually indicated the formation of lamellar (Lα) liquid crystalline phase.42
 | ||
| Fig. 4 Polarized optical micrographs for BPS-5 in glycerol at different concentrations at 25 °C. From (a) to (c), CBPS-5 = 50%, 70% and 90%. | ||
Such possible Lα phase structures were further characterized by their SAXS profiles. As shown in Fig. S1† for upper anisotropic one in two-phase separated samples at CBPS-5 = 25% and 40%, three Bragg scattering peaks with the relative scattering factor q positions of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio could be obtained, indicating definitely the formation of Lα phases.43,44 At concentrations higher than 50% in the uniform single phase samples, we still observed three or more Bragg scattering peaks with their relative scattering factor q positions of 1
3 ratio could be obtained, indicating definitely the formation of Lα phases.43,44 At concentrations higher than 50% in the uniform single phase samples, we still observed three or more Bragg scattering peaks with their relative scattering factor q positions of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio corresponding to the formation of Lα phase as seen in Fig. 5a. Based on the Bragg equation, d = 2π/q1, the repeated lamellae distance (d) could be derived. When the CBPS-5 was increased from 50% to 90%, the d values were contracted from 8.02 to 6.02 nm.
3 ratio corresponding to the formation of Lα phase as seen in Fig. 5a. Based on the Bragg equation, d = 2π/q1, the repeated lamellae distance (d) could be derived. When the CBPS-5 was increased from 50% to 90%, the d values were contracted from 8.02 to 6.02 nm.
 | ||
| Fig. 5 SAXS patterns for uniform single phase of BPS-5 in glycerol at different concentrations (a) and at four concentrations with two sets scattering peaks (b) at 25 °C. | ||
It was interested that two sets of scattering peaks were observed in the sample at CBPS-5 = 70% (Fig. 5a). Further SAXS characterization at concentrations around 70% were then carried out with the results shown in Fig. 5b. Though not so obvious, it could be still noted that the separation of Bragg peaks was initiated at 65%, especially at the 2nd peak. In both sets of scattering peaks, their relative scattering factor q positions were still of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratios, indicating a coexistence of two kinds of lamellar phases in this concentration region. By carefully checking the 1st peaks in these profiles, the scattering factors in set 1 were closer to those of lamellar phase before the occurrence of another set SAXS pattern. While the scattering factors in set 1′ were closer to those of lamellar phases at higher concentrations larger than 80%. The relatively big difference between the lamellae repeat spacings (d) at 60% (7.50 nm) and 80% (6.66 nm) provided certain clue to explain this phenomenon. The change of d value with the volume fraction of solvophobic component was plotted according to the method described previously27 with the result shown in Fig. S2.† A disruption was found at CBPS-5 around 70%. Therefore, the molecular packing inside both lamellar phases should be in different manners. Considering the existence of three-dimensional hydrogen-bonded network in glycerol,45 and the possible hydrogen bondings either between glycerol and PEO chains in BPS-5 or just between PEO chain themselves, the lamellar phase at CBPS-5 less than 60% should be organized with more glycerol incorporated between PEO chains, which were then more extended. With the decreasing of glycerol amount, the interaction between PEO chains was enhanced to induce them more interdigitated and therefore an obvious reduction of d value.
3 ratios, indicating a coexistence of two kinds of lamellar phases in this concentration region. By carefully checking the 1st peaks in these profiles, the scattering factors in set 1 were closer to those of lamellar phase before the occurrence of another set SAXS pattern. While the scattering factors in set 1′ were closer to those of lamellar phases at higher concentrations larger than 80%. The relatively big difference between the lamellae repeat spacings (d) at 60% (7.50 nm) and 80% (6.66 nm) provided certain clue to explain this phenomenon. The change of d value with the volume fraction of solvophobic component was plotted according to the method described previously27 with the result shown in Fig. S2.† A disruption was found at CBPS-5 around 70%. Therefore, the molecular packing inside both lamellar phases should be in different manners. Considering the existence of three-dimensional hydrogen-bonded network in glycerol,45 and the possible hydrogen bondings either between glycerol and PEO chains in BPS-5 or just between PEO chain themselves, the lamellar phase at CBPS-5 less than 60% should be organized with more glycerol incorporated between PEO chains, which were then more extended. With the decreasing of glycerol amount, the interaction between PEO chains was enhanced to induce them more interdigitated and therefore an obvious reduction of d value.
To get more detailed structural information of the lamellar phase, the volume fraction of solvophobic components (Φa), the solvophobic domain thickness (dc), the solvophilic thickness (dg), and the effective cross-sectional area per molecule at the surfactant/glycerol interface (AS) were calculated based on the following equations and relations,24,43
| CBPS-n (wt%) | q1 (nm−1) | d (nm) | Φa (vol%) | dc (nm) | dg (nm) | AS (nm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60% | 0.837 | (0.734) | 7.50 | (8.56) | 0.41 | (0.32) | 1.53 | (1.37) | 4.44 | (5.82) | 0.42 | (0.47) |
| 70% | 0.870 | (0.798) | 7.22 | (7.87) | 0.47 | (0.37) | 1.69 | (1.46) | 3.84 | (4.95) | 0.38 | (0.44) |
| 80% | 0.943 | (0.828) | 6.66 | (7.58) | 0.52 | (0.41) | 1.75 | (1.56) | 3.16 | (4.46) | 0.36 | (0.41) |
| 90% | 1.043 | (0.859) | 6.02 | (7.31) | 0.58 | (0.46) | 1.75 | (1.68) | 2.53 | (3.95) | 0.36 | (0.38) |
The change profile of d with Φa in BPS-5 system had been plotted in Fig. S2,† which presented two approximately linear relationship between log(d) and log(Φa), indicating two lamellar structures as discussed before. For BPS-10, such a profile behaved more like only one linear relationship for a one-dimensional swelling scheme44,46 as shown in Fig. S4,† consistent with a single lamellar structure in LLC region as discussed before. Changes of three other structural parameters with the volume fraction of glycerol (Φg) were also plotted and shown in Fig. S5.† Only little changes for dc and AS values could be seen. The a little higher solvophobic domain thickness (dc) in BPS-5 system and a little bigger solvophilic thickness (dg) in BPS-10 case reflected respectively an enhanced solvophobic volume in BPS-5 molecular packing and an increased solvophilic interactions due to the longer EO chains in BPS-10.47 In addition, the reduced AS and d values compared to those in EAN48 confirmed a more dense packing of BPS-10 in glycerol, which was consistent with the higher Gordon parameter of glycerol than EAN.
3.3 Rheological properties of LLC in BPS-5 and BPS-10 systems
To get more information on molecular interactions inside the aggregate, the viscoelastic properties of formed LLC phases at different concentrations were studied by rheological measurements. The obtained results indicated a shear-thinning rheology behavior for the Lα phase of BPS-5 at higher concentrations (50% to 90%). The apparent viscosity was increased with increasing CBPS-5 and higher than those of the conventional nonionic surfactant like CnEOm,49,50 at low shear rates, which might be attributed to the stronger interactions between steroid rings.Fig. 6 presented the results from the dynamic oscillatory experiments. As can be seen that, both the storage (G′) and loss (G′′) moduli were increased with increasing the sweep frequency, indicating a viscoelastic liquid character.34 Meanwhile, the complex viscosity (η*) values were decreased with elevating the sweep frequency. All these phenomena were characteristic for the formation of Lα phase of BPS-5 in glycerol.51 It could be noted also in Fig. 6a that for the sample at CBPS-5 of 60%, the G′ was higher in the low frequency region but lower in the high frequency region compared to G′′. However, when CBPS-5 was increased to 80% or higher, G′ was always larger than G′′ at the whole measured frequency range, indicating only an elastic behavior, which was characteristic for gel-like aggregate.52,53 Meanwhile, the complex viscosity was also enhanced as seen in Fig. 6b. All these results reflected a concentration induced denser packing of BPS-5 molecules in glycerol, which was in accordance with the deduction from SAXS results.
 | ||
| Fig. 6 Variation of storage and loss moduli and the complex viscosity as a function of shear frequency at different CBPS-5: (a) 60% and (b) 80%. | ||
Similar rheological properties were observed for the Lα phase samples of BPS-10 in glycerol with the results shown in Fig. S6 and S7.† But, the apparent viscosity of BPS-10 Lα phase was lower than that of BPS-5 at high concentrations, denoting a looser packing of BPS-10 and being consistent with the increased AS values. However, these viscosity values were still about ten times higher than those of BPS-10 in EAN and [Bmim]BF4,28 and the moduli values in glycerol were also much higher than those in both ionic liquids and water.23,28 The reason should be attributed to the stronger interactions between BPS-10 and glycerol.
Based on the results discussed above, the preliminary phase diagrams of BPS-n/glycerol binary systems with five or ten oxyethylene units could be mapped and shown in Fig. 7. At lower concentrations, micelles coexisted with the Lα phase in both binary systems. With increasing BPS-n concentration, the uniform sticky Lα phases could be formed in both BPS-5 and BPS-10 systems. There existed two kinds of lamellar phases (Lα and L′α) at high concentrations of BPS-5, while only one lamellar phase could be formed for BPS-10 in glycerol at high concentrations. It was noted that the initial concentration for lamellar phase appearance was reduced in BPS-n/glycerol binary systems in compared with those LLC systems reported for BPS-n in water or [Bmim]BF4,23,27 indicating the unusual self-assembling capability presented in glycerol.
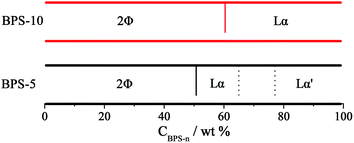 | ||
| Fig. 7 Phase diagram for BPS-n (n = 5, 10)/glycerol binary systems at 25 °C. 2Φ denotes a two-phase coexistence. | ||
3.4 Molecular interactions between BPS-n and glycerol
What causes the presence of two Lα phases of BPS-5 in glycerol and what is the origin of the good self-assembling capability of glycerol? To answer these questions, the main driving forces during the LLC phase formation should be depicted. In the light of the unique structural character of BPS-n molecules with the solvophilic PEO block and the solvophobic steroid nucleus, these molecules should adopt well-ordered molecular arrangements inside the aggregates. In our previous work, the strong solvophobic interactions between bulky ring groups have been demonstrated as the main driving force on the formation of Lα phase in BPS-n/ILs system, and the hydrogen bonding between PEO blocks and ILs was another important driving force.27,54Using glycerol as solvent, however, the contribution from hydrogen bonding became more obvious because of the presence of more hydroxyl groups in glycerol. In addition, the H-bonding mean lifetimes of glycerol which was even longer than that in water,47,55 might induce the stronger solvophobic effect of BPS-n than in water. Thus, we could speculate that the hydrogen bonding between PEO block and glycerol molecules was another source of driving force during the formation of LLCs. This point could be confirmed by the FTIR spectra for BPS-5/glycerol system with the results shown in Fig. 8. In the BPS-5/glycerol binary system, the stretching vibrations of the hydroxyl groups at 3528 cm−1 in pure glycerol and the C–O–C stretching vibration of PEO groups at 1109 cm−1 were both shifted to lower wavenumbers. These frequency variations denoted the formation of hydrogen bonding between the hydroxyl group of glycerol solvent and the oxygen atom of PEO blocks.42 Similar FTIR spectra changes were obtained for BPS-10 in glycerol with the results shown in Fig. S8.† Based on the facts that the Lα phase repeat distances at the higher concentration like 90% (6.02 or 7.31 nm for BPS-5 or BPS-10 systems) were smaller than their corresponding bilayer thicknesses (5.28 × 2 or 6.87 × 2 for BPS-5 or BPS-10),48 it was reasonable to suppose an interdigitated antiparallel model (shown in Fig. S9†) for the molecular packing of BPS-n in Lα phase, where only PEO chains were overlapped with each other via hydrogen bonds between the hydroxyl group of glycerol and the oxygen atom of PEO blocks.
Then, it was a little strange that only the Lα phase was formed in BPS-n/glycerol system. With the Gordon parameter value close to that of formamide and higher than that of EAN, the BPS-n surfactants should form richer morphological aggregates in glycerol but not only the lamellar one. This fact suggested that the moderate solvophilic head-solvent interaction and geometric constraints were other important factors to control the final aggregate morphology.39
4. Conclusions
The aggregation behaviors of two phytosterol ethoxylate surfactants (BPS-5 and BPS-10) in glycerol were investigated in this work. Both of them formed micelles with their CMC values in between those of water and ionic liquids (EAN or [Bmim]BF4), reflecting a better capability of glycerol as self-assembling medium than ionic liquids. The reason was due to its similarity to water and EAN on the ability to donate protons to form hydrogen bonding networks. The Lα phases were formed from BPS-5 and BPS-10. However, BPS-n in glycerol exhibited better aggregation behaviors compared to those in water or ionic liquids, which might be attributed to their stronger hydrogen-bonded network and much longer hydrogen bonding lifetime. The main driving forces on the formation of the Lα phases were the interactions of the steroid rings and hydrogen bonding effects. The geometric constraints and head-solvent interactions were demonstrated to be the other factors. For BPS-5 system, the delicate balance between these interactions induced two kinds of lamellar phases (Lα and L′α) at concentrations around 70%. The deductions and conclusions derived here should be useful supplements to the aggregation behaviors of steroid surfactant in non-aqueous solvents and therefore extend their applications in water-free self-assemblies, which will increase the potential applications of BPS-n surfactant in the future.Acknowledgements
We are thankful for the financial supports from the National Natural Science Foundation of China (20973104 and 21373127) and the Specialized Research Fund for the Doctoral Program of Higher Education (20130131110010).References
- Novel Surfactants: Preparation Applications and Biodegradability, ed. K. Holmberg, Marcel Dekker, Inc., New York, 2nd edn, 2003 Search PubMed.
- B. M. Folmer and M. Nyden, Langmuir, 2008, 24, 6441 CrossRef CAS PubMed.
- K. Miyajima, T. Lee and M. Nakagaki, Chem. Pharm. Bull., 1984, 32, 3670 CrossRef CAS.
- D. Meissner, I. Grassert, G. Oehme, G. Holzhüter and V. Vill, Colloid Polym. Sci., 2000, 278, 364 CAS.
- C. Müller-Goymann, Pharm. Res., 1984, 1, 154 CrossRef PubMed.
- I. F. Uchegbu, D. McCarthy, A. Schätzlein and A. T. Florence, STP Pharma Sci., 1996, 6, 33 Search PubMed.
- S. Beugin, K. Edwards, G. Karlsson, M. Ollivon and S. Lesieur, J. Biophys., 1998, 74, 3198 CrossRef CAS.
- J. Bouwstra, D. van Hall, H. Hofland and H. Junginger, Colloids Surf., A, 1996, 178, 263 Search PubMed.
- E. F. Marques, H. Edlund, C. L. Mesa and A. Khan, Langmuir, 2000, 16, 5178 CrossRef CAS.
- H. Amenitsch, H. Edlund, A. Khan, E. F. Marques and C. La Mesa, Colloids Surf., A, 2003, 213, 79 CrossRef CAS.
- H. Edlund, A. Khan and C. L. Mesa, Langmuir, 1998, 14, 3691 CrossRef CAS.
- H. Soderlund, J. Sjoblom and T. Warnheim, J. Dispersion Sci. Technol., 1989, 10, 131 CrossRef.
- D. Libster, A. Aserin, D. Yariv, G. Shoham and N. Garti, Colloids Surf., B, 2009, 74, 202 CrossRef CAS PubMed.
- T. Teshigaware, R. Miyahara, T. Fukuhara and T. Oka, J. Oleo Sci., 2009, 58, 27 CrossRef.
- H. Sakai, T. Saitoh, T. Endo, K. Tsuchiya, K. Sakai and M. Abe, Langmuir, 2009, 25, 2601 CrossRef CAS PubMed.
- K. Sakai, Y. Onuma, K. Torigoe, S. Biggs, H. Sakai and M. Abe, Langmuir, 2011, 27, 3244 CrossRef CAS PubMed.
- S. C. Sharma, L. K. Shrestha, K. Tsuchiya, K. Sakai, H. Sakai and M. Abe, J. Phys. Chem. B, 2009, 113, 304 Search PubMed.
- R. G. Shrestha, L. Abezgauz, D. Danino, K. Sakai, H. Sakai and M. Abe, Langmuir, 2011, 27, 12877 CrossRef CAS PubMed.
- R. G. Shrestha, S. C. Sharma, K. Sakai, H. Sakai and M. Abe, Colloid Polym. Sci., 2012, 290, 339 CAS.
- S. C. Sharma, L. K. Shrestha, K. Sakai, H. Sakai and M. Abe, Colloid Polym. Sci., 2010, 288, 405 CAS.
- R. G. Shrestha, K. Sakai, H. Sakai and M. Abe, J. Phys. Chem. B, 2011, 115, 2937 CrossRef CAS PubMed.
- B. M. Folmer, M. Svensson, K. Holmberg and W. Brown, J. Colloid Interface Sci., 1999, 213, 112 CrossRef CAS PubMed.
- B. M. Folmer, J. Colloid Interface Sci., 2003, 103, 99 CrossRef CAS.
- C. Rodriguez, N. Naito and H. Kunieda, Colloids Surf., A, 2001, 181, 237 CrossRef CAS.
- M. K. Hossain, D. P Acharya, T. Sakai and H. Kunieda, J. Colloid Interface Sci., 2004, 277, 235 CrossRef CAS PubMed.
- H. Sakai, T. Saitoh, T. Misono, K. Tsuchiya, K. Sakai and M. Abe, J. Oleo Sci., 2012, 61, 135 CrossRef CAS PubMed.
- X. Yue, X. Chen, X. D. Wang and Z. H. Li, Colloids Surf., A, 2011, 392, 225 CrossRef CAS.
- X. Yue, X. Chen and Q. T. Li, J. Phys. Chem. B, 2012, 116, 9439 CrossRef CAS PubMed.
- R. Palepu, H. Gharibi, D. M. Bloor and E. Wyn-Jones, Langmuir, 1993, 9, 110 CrossRef CAS.
- C. Carnero Ruiz, J. A. Molina-Bolívar, J. Aguiar, G. MacIsaac, S. Moroze and R. Palepu, Langmuir, 2001, 17, 6831 CrossRef.
- C. Seguin, J. Eastoe, R. Clapperton, R. K. Heenan and I. Grillo, Colloids Surf., A, 2006, 282–283, 134 CrossRef.
- K. M. Glenn, S. Moroze, S. C. Bhattacharya and R. M. Palepu, J. Dispersion Sci. Technol., 2005, 26, 79 CrossRef CAS.
- X. Yue, X. Chen, Q. T. Li and Z. H. Li, Langmuir, 2013, 29, 11013 CrossRef CAS PubMed.
- B. Sarkar, S. Lam and P. Alexandridis, Langmuir, 2010, 26, 10532 CrossRef CAS PubMed.
- J. Penfold, E. Staples, I. Tucker and P. Cummins, J. Colloid Interface Sci., 1997, 185, 424 CrossRef CAS PubMed.
- M. A. EI-nokaly, L. D. Ford and S. E. Friberg, J. Colloid Interface Sci., 1981, 84, 228 CrossRef.
- H. D. Dorf1er and A. Senst, Colloid Polym. Sci., 1993, 271, 173 Search PubMed.
- X. Auvray, C. Petipas, R. Anthore, I. Rico and A. Lalles, J. Phys. Chem., 1989, 93, 7458 CrossRef CAS.
- X. Auvray, T. Perche, R. Anthore, C. Petipas, I. Rico and A. Lattes, Langmuir, 1991, 7, 2385 CrossRef CAS.
- G. D’Errico, D. Ciccarelli and O. Ortona, J. Colloid Interface Sci., 2005, 286, 747 CrossRef PubMed.
- T. L. Greaves and C. J. Drummond, Chem. Soc. Rev., 2008, 37, 1709 RSC.
- F. B. Rosevear, J. Am. Oil Chem. Soc., 1954, 31, 628 CrossRef CAS.
- H. Kunieda, G. Umizu and Y. Yamaguchi, J. Colloid Interface Sci., 1999, 218, 88 CrossRef CAS PubMed.
- G. D. Zhang, X. Chen, Y. R. Zhao, Y. Z. Xie and H. Y. Qiu, J. Phys. Chem. B, 2007, 111, 11708 CrossRef CAS PubMed.
- J. A. Padro, L. Saiz and E. Guardia, J. Mol. Struct., 1997, 416, 243 CrossRef CAS.
- P. Ekwall, L. Mandell and K. Fontell, J. Colloid Interface Sci., 1969, 29, 639 CrossRef CAS PubMed.
- R. Atkin, S. M. C. Bobillier and G. G. Warr, J. Phys. Chem. B, 2010, 114, 1350 CrossRef CAS PubMed.
- M. A. Lopez-Quintela, A. Akahane, C. Rodriguez and H. Kunieda, J. Colloid Interface Sci., 2002, 247, 186 CrossRef CAS PubMed.
- F. M. Ma, X. Chen, Y. R. Zhao, X. D. Wang, Q. H. Li, C. Lv and X. Yue, Langmuir, 2010, 26, 7802 CrossRef CAS PubMed.
- P. Versluis, J. C. van de Pas and J. Mellema, Langmuir, 1997, 13, 5732 CrossRef CAS.
- R. Mezzenga, C. Meyer, C. Servais, A. I. Romoscanu, L. Sagalowicz and R. C. Hayward, Langmuir, 2005, 21, 3322 CrossRef CAS PubMed.
- T. Durrschmidt and H. Hoffmann, Colloid Polym. Sci., 2001, 279, 1005 CAS.
- J. Zhang, A. X. Song, Z. B. Li, G. Y. Xu and J. C. Hao, J. Phys. Chem. B, 2010, 114, 13128 CrossRef CAS PubMed.
- L. Y. Wang, X. Chen, Y. C. Chai, J. C. Hao, Z. M. Sui, W. C. Zhuang and Z. W. Sun, Chem. Commun., 2004, 24, 2840 RSC.
- E. Guardia, J. Marti, J. A. Padro, L. Saiz and A. V. Komolkin, J. Mol. Liq., 2002, 96–97, 3 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The aggregation behaviors of BPS-10 in glycerol and more structural information of the lamellar phase of BPS-5 were characterized by POM, SAXS, FTIR and rheology measurement with the results shown in supporting information. See DOI: 10.1039/c5ra21446g |
| This journal is © The Royal Society of Chemistry 2015 |





