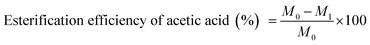Synthesis, characterization and properties of Ce-modified S2O82−/ZnAl2O4 solid acid catalysts
Jun-Xia Wang*,
An-Qi Wang,
Yu-Lin Xing,
Zheng-Xin Zhu,
Xiu-Ling Wu,
Yong-Qian Wang and
Li-Xia Yang
Faculty of Materials Science and Chemistry, Engineering Research Center of Nano-Geomaterials of Ministry of Education, China University of Geosciences, Wuhan 430074, China. E-mail: wjx76@sina.com; Fax: +86-27-87407079; Tel: +86-27-87407079
First published on 2nd December 2015
Abstract
A new spinel solid acid catalyst of S2O82−/ZnAl2O4-x wt% Ce was simply prepared by modifying S2O82−/ZnAl2O4 with Ce for acid catalysis of acetic acid and n-butanol. The prepared catalysts were characterized by means of XRD, IR, TG, XPS, NH3-TPD, SEM and N2-physisorption. The experimental results showed S2O82−/ZnAl2O4-x wt% Ce solid acid catalysts belonged to the spinel-type ZnAl2O4 structure. The addition of Ce played a key role in stabilizing the surface sulfur species and consequently increasing the acid strength of S2O82−/ZnAl2O4-x wt% Ce. The appropriate modification of Ce was 4 wt% and S2O82−/ZnAl2O4-4 wt% Ce catalyst had 95.9% esterification efficiency under the optimum reaction conditions. Compared with unmodified S2O82−/ZnAl2O4 catalyst, S2O82−/ZnAl2O4-4 wt% Ce solid acid catalyst showed much better reusability, which could remain above 80% esterification even after being used for six times. The loss of sulfur species on the surface of S2O82−/ZnAl2O4-4 wt% Ce solid acid was one of the essential reasons for its deactivation during the acid catalyzed reaction.
1. Introduction
In recent years, acid catalysts have been widely used in many acid catalytic reactions, including dehydration, isomerization, acylation, esterification, alkylation and polymerization, etc. Conventional industrial acid catalysts, such as inorganic acids (e.g. H3PO4, HCl, H2SO4, etc.) and Lewis acids (e.g. AlCl3, TiCl4, etc.), have unavoidable drawbacks because of their severe corrosivity and environmental problems. To overcome these disadvantages, a number of heterogeneous solid acid catalysts, such as ion exchange resin, sulfonated-carbon materials, zeolites, heteropoly acids, niobium oxide, sulphated metal oxides (SO42−/MxOy), etc., have been studied because of their significant advantages of easy recovery, less waste, less corrosion, and environmental safety.1–5 Nowadays, solid acid catalysis has been tested for many organic reactions, such as esterification, sugar dehydration, n-alkanes isomerization, condensation reaction, acetalization reaction, and so forth.6–9 SO42−/MxOy is an interesting class of solid acid catalyst because of its unique advantages.10,11 For example, it has been demonstrated that it is easy to synthesize and presents other advantages of better thermal stability, stronger acidity and higher catalytic activity in many kinds of organic reactions even under very mild conditions. However, SO42−/MxOy solid acid catalysts, including two representative systems of SO42−/ZrO2 and SO42−/TiO2, usually surfer from low stability and rapid deactivation, which limit their promising practical applications.12,13 To overcome these drawbacks, numerous approaches have been employed over the past several decades. Among them, the modification of SO42−/MxOy with other metallic ions is typically considered as an effective method to greatly improve the acid sites dispersion, stabilize the surface sulfur species and enhance the stability of SO42−/MxOy solid acid catalysts.14–18 However, crystal structure transformation will inevitably happen in the process of modification SO42−/MxOy solid acid with other metallic ions. Moreover, this phenomenon is difficult to control and leads to a negative effect on the catalytic performances of SO42−/MxOy solid acid catalysts.19–21 So, the quest for new superior systems that avoids the above disadvantages of SO42−/MxOy has led to exploration of alternative metal oxides as a substitute for synthesis of SO42−/MxOy solid acid catalysts. In our preliminary experiment, we had identified that composite oxide spinel ZnAl2O4 could be successfully used in synthesis of S2O82−/ZnAl2O4 spinel solid acid, which had exhibited high catalytic activity in the esterification of n-butyl acetate. Particularly, S2O82−/ZnAl2O4 had the prominent advantages of stable structure owing to its single spinel crystal shape.22 However, for unmodified S2O82−/ZnAl2O4 solid acid catalyst, there were still similar disadvantage of the relatively short lifetime and low reusability with other SO42−/MxOy solid acid catalysts. Therefore, directions for further improving its catalytic properties include modifying S2O82−/ZnAl2O4 with other promoters.Based on the above background, the present work makes an attempt on modifying S2O82−/ZnAl2O4 with Ce. The main aim of this paper is to investigate the effect of Ce modification on acidic properties, the catalytic activities and the stability of S2O82−/ZnAl2O4 spinel solid acid in detail. For this purpose, a new spinel solid acid catalyst of S2O82−/ZnAl2O4-x wt% Ce was simply prepared by modifying S2O82−/ZnAl2O4 with Ce for acid catalysis of acetic acid and n-butanol in this paper. In addition, synthesis conditions of S2O82−/ZnAl2O4-4 wt% Ce solid acid catalyst were optimized, including reaction times, acetic acid/n-butanol ratio and catalyst amounts. In the meantime, the reusability of S2O82−/ZnAl2O4-4 wt% Ce solid acid catalyst was also evaluated. The catalyst structure and the acidic activity of S2O82−/ZnAl2O4-x wt% Ce solid acid catalysts were characterized by XRD, IR, TG, NH3-TPD, XPS, SEM and N2-physisorption techniques. Up to now, there are few reports about the reasons for deactivation of SO42−/MxOy solid acid catalysts. However, it is of great importance in the evaluation of catalyst lifetime. Therefore, we had an attempt to understand of the deactivation of S2O82−/ZnAl2O4-4 wt% Ce solid acid during the reaction, with the aim to provide the reference data for the further study of SO42−/MxOy solid acid catalyst. There are only few studies on concerning S2O82−/ZnAl2O4-x wt% Ce solid acid catalysts, which are expected to act as a promising catalyst system and have certain application prospect in the acid catalytic reaction.
2. Experimental
2.1 Catalysts preparation
Solid acid catalysts S2O82−/ZnAl2O4-x wt% Ce (x = 0, 2, 4, 6, 8, 10) were prepared as follows: 15.004 g Al(NO3)3·9H2O, 5.950 g Zn(NO3)2·6H2O and stoichiometry amounts of Ce(NO)3·6H2O were dissolved in absolute ethyl alcohol, followed by adding 5 wt% (∼1.048 g) of polyethylene glycol to the ethyl alcohol solution with magnetic stirring at room temperature for 4 h. The obtained mixture was evaporated with further stirring for 2 h in a water bath at 60 °C to get the sol. The sol was dried and was grounded into a fine powder. Then, the powder was calcined at 600 °C for 5 h in air sequentially to obtain the spinel ZnAl2O4-x wt% Ce. The ZnAl2O4-x wt% Ce precursors were then sulfated for 12 h by impregnating with 1.50 mol L−1 (NH4)2S2O8 solution on the proportion of 10 mL g−1 solution to 1 g precursors. Finally, after having been filtered and dried, the samples were calcined at 550 °C for 5 h in air, which subsequently obtained the S2O82−/ZnAl2O4-x wt% Ce catalysts.2.2 Catalysts characterization
The solid phases of the calcined catalysts were determined by using the X-ray powder diffraction (XRD) (Bruker AXS D8-Focus X diffractometer), using CuKα radiation at 40 kV and 40 mA; IR spectrum of the catalysts was recorded by a Nicolet 6700 IR spectrometer using the KBr pellet technique; thermogravimetric analysis (TG) was performed on a STA-409PC thermo analyzer in the temperature range of 30–1000 °C with a heating rate of 20 °C min−1; Scanning Electron Microscopy (SEM) was performed on a SU 8010N electron microscope with an acceleration voltage of 10 kV; the X-ray photoelectron spectroscopy (XPS) was performed in a VG Multilab 2000; the NH3 temperature programmed desorption (NH3-TPD) experiments were carried out using a Micromeritics AutoChem II 2920 quipped with a TCD detector; N2 adsorption analysis was carried out using micromeritics (ASAP-2020) at liquid nitrogen temperature (77 K), and the surface area was calculated by the BET method and the pore size distribution was obtained from the adsorption isotherm by the BJH method.2.3 Catalytic activity test
The catalytic activities of the catalysts were tested in a three-necked flask equipped with a magnetic stirrer, a thermometer and a refluxing condenser tube under atmospheric pressure. The reaction conditions were as follows: the range of reaction temperatures was 115–118 °C, the molar ratio of acetic acid to n-butanol was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; the reaction time was 1.0–4.0 h; the catalyst amount was 0–2.21% (percentage content of the reaction mixture). The initial and residual acid was determined by means of titration. The esterification efficiency of acetic acid can be calculated using the following equation (by the method of GB1668-81):
5; the reaction time was 1.0–4.0 h; the catalyst amount was 0–2.21% (percentage content of the reaction mixture). The initial and residual acid was determined by means of titration. The esterification efficiency of acetic acid can be calculated using the following equation (by the method of GB1668-81):where M0 is the acid value before reaction and M1 is the acid value after reaction. 0.50 mL initial or final reaction mixture was added in 20.00 mL absolute alcohol and titrated by 0.10 mol L−1 NaOH solution using phenolphthalein as an indicator.
In order to test the catalyst lifetime, S2O82−/ZnAl2O4-4 wt% Ce was repeatedly used for the batch reaction process under the optimum synthesis conditions. After each catalytic evaluation was finished, the catalyst was recovered by filtering and drying and reused for the next evaluation.
3. Results and discussion
3.1 Catalytic activities
Table 1 showed the effect of x value on the catalytic activity of S2O82−/ZnAl2O4-x wt% Ce in esterification of acetic acid with n-butanol. For comparison, the catalytic activities of the support of ZnAl2O4 and ZnAl2O4-4 wt% Ce were also provided in Table 1. It was found that both ZnAl2O4 and ZnAl2O4-4 wt% Ce showed lower catalytic activity with less than 50% esterification efficiency. However, S2O82−/ZnAl2O4-x wt% Ce catalysts all exhibited significantly high catalytic activities with above 90% esterification efficiency. According to the IR results, their higher catalytic activities of S2O82−/ZnAl2O4-x wt% Ce catalysts were attributed to the formation of the acid structures between sulfuric groups and metal ions. By comparison of S2O82−/ZnAl2O4, S2O82−/ZnAl2O4-x wt% Ce (x = 2–10) catalysts presented better catalytic activities. This result may be caused by the following reasons. First, the cerium addition changed the chemical state of the exterior atom in the spinel lattice of the samples, which was confirmed by the results of XRD and XPS. Correspondingly, the polarization degree of surface elements was increased. As a result, the addition of Ce enhanced the acid strength of S2O82−/ZnAl2O4-x wt% Ce, which was further proved by NH3-TPD and TG techniques. Secondly, the addition of cerium could strengthen the interaction between S2O82− and metallic ions, which is beneficial to preserve sulfate ions on the surface of catalysts and prevent the loss of S2O82−.20,23,24 This was coincident with the experimental results of the reusability, IR and XPS studies. Based on the above analysis, we could assume that the addition of cerium played an important role in the improvement of acidic property and the catalytic activities of the samples. The similar results were also reported by other literatures. For example, Xiao et al. prepared cerium-doped mesoporous TiO2 nanofiber (SO42−/Cex/TiO2) solid acid catalysts, and discovered that doping Ce into TiO2 resulted in the increase of total acidity.16 Yet, it was worth noting that excess cerium as an assistant component might reduce the number of S2O82− bonding to the surface of metal ions, thereby the acid strength and catalytic activities of the catalysts would drop accordingly. As shown in Table 1, the appropriate addition of Ce was 4 wt% and S2O82−/ZnAl2O4-4 wt% Ce exhibited the highest catalytic activity with maximum 95.9% esterification efficiency. Based on the results of NH3-TPD, this result might be owing to its strongest acid strength and its maximum amount of acid sites in all samples.| Catalysts | x value | Esterification efficiency (%) |
|---|---|---|
a The range of reaction temperature was 115–118 °C; the molar ratio of acetic acid to n-butanol was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; the amount of catalyst was 1.55%; the reaction time was 3 h. 3; the amount of catalyst was 1.55%; the reaction time was 3 h. |
||
| S2O82−/ZnAl2O4-x wt% Ce | 0 | 91.4 |
| 2 | 94.3 | |
| 4 | 95.9 | |
| 6 | 95.4 | |
| 8 | 94.8 | |
| 10 | 94.8 | |
| ZnAl2O4-x wt% Ce | 0 | 45.2 |
| ZnAl2O4-x wt% Ce | 4 | 44.5 |
It is well known that the catalyst amounts are closely related with the number of acid center on the surface of the solid acid catalysts, which play an important role in the catalytic activity of the catalysts. Thus, the reaction was studied with different catalyst amounts of S2O82−/ZnAl2O4-4 wt% Ce. As shown in Table 2, the esterification efficiency was less than 40% when the reaction was carried out without catalyst. The esterification efficiency was obviously improved with the addition of the catalyst. In the meantime, the esterification efficiency was increased with catalyst amounts increasing from 0.37 to 1.55%, which might be attributed to the increase of the number active sites with increasing catalyst amounts. However, the excess acid amount might promote the occurrence of the reverse reaction.25 Accordingly, the esterification efficiency was reduced slightly when the catalyst amount exceeded 1.55%. Thus, the optimum catalyst amount was considered to be 1.55% with the maximum 95.9% esterification efficiency.
| Catalyst | Amounta | Time (h) | a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nb ratio nb ratio |
Esterification efficiency (%) |
|---|---|---|---|---|
| a The amount was calculated on the basis of total weight of the reactants.b The molar ratio of acetic acid to n-butanol. | ||||
| S2O82−/ZnAl2O4-4 wt% Ce | 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
57.1 |
| 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
86.2 | |
| 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
95.9 | |
| 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
95.9 | |
| 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
95.9 | |
| 0.00 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
38.0 | |
| 0.37 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
79.6 | |
| 0.73 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
83.4 | |
| 1.14 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
88.7 | |
| 1.85 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
95.8 | |
| 2.21 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
95.1 | |
| 1.55 | 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
86.6 | |
| 1.55 | 2.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91.8 | |
| 1.55 | 2.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
94.4 | |
| 1.55 | 3.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
96.5 | |
| 1.55 | 4.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
96.8 | |
Table 2 gave the effects of the molar ratio of acetic acid to n-butanol on the esterification efficiency of S2O82−/ZnAl2O4-4 wt% Ce. The esterification efficiency was increased obviously with increasing the molar ratio of acetic acid to n-butanol from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. This result might be that excess n-butanol was usually beneficial to push the reversible reaction forward, which resulted in the increase of the esterification efficiency. However, a mass of n-butanol might dilute the concentration of the acid, so there was no obvious change in the esterification efficiency when the molar ratio of acetic acid to n-butanol was beyond 1
3. This result might be that excess n-butanol was usually beneficial to push the reversible reaction forward, which resulted in the increase of the esterification efficiency. However, a mass of n-butanol might dilute the concentration of the acid, so there was no obvious change in the esterification efficiency when the molar ratio of acetic acid to n-butanol was beyond 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.26 Thereby, the molar ratio of 1
3.26 Thereby, the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was sufficient to achieve a high catalytic activity.
3 was sufficient to achieve a high catalytic activity.
The reaction time is one of the most important factors that affect the catalytic activity of the catalysts. The effects of the reaction time on the catalytic activity of S2O82−/ZnAl2O4-4 wt% Ce were examined. As listed in Table 2, the longer reaction time would lead to the esterification rate increase rapidly when the reaction time was less than 3 h. And then, the esterification efficiency had leveled off at around 96% in longer duration of reaction. So, 3 h was sufficient for the completion of the esterification reaction.
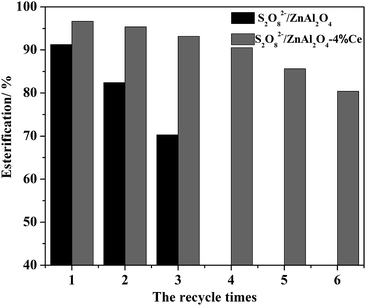
It is well known that SO42−/MxOy solid acid catalysts commonly suffer from rapid deactivation and low service life despite of their high initial activities, which is mainly owing to the loss of sulfur species as well as carbon deposition.9 For example, Shi had reported that the catalytic activities of sulfate-promoted iron oxide dropped from 84.48% to 51.48% after 10 recycles in the n-butyl acetate esterification.27 In fact, deactivation is a general problem and a rather complicated phenomenon in heterogeneous catalysis, which is also the main problem faced by SO42−/MxOy solid acid catalysts.2 Thus, the reusability and its improvement are of great importance in the study of SO42−/MxOy solid acid catalysts. In view of this point, S2O82−/ZnAl2O4 and S2O82−/ZnAl2O4-4 wt% Ce solid acid catalysts were recycled to study the stability of the catalysts under the same synthesis conditions. The results were presented in Fig. 1. It could be seen clearly that the addition of Ce played a very important role in improving the lifetime and the stability of the catalyst. Compared with S2O82−/ZnAl2O4, S2O82−/ZnAl2O4-4 wt% Ce solid acid catalyst showed the obviously better reusability, which remained above 80% esterification even after being used for six times. This result might be attribute to its excellent structural stability and excellent resistance to the loss of the sulfur species. This result was also in agreement with other literature.28
Table 3 demonstrated the comparison of S2O82−/ZnAl2O4-4 wt% Ce with other reported solid acid catalysts. The catalytic activity of S2O82−/ZnAl2O4-4 wt% Ce is comparable with the series of SO42−–Ce0.02/TiO2,16 mesoporous materials catalyst,29 and other type solid acid catalysts30–32 in this esterification reaction. However, crystal structure transformation was generally occurred in the synthesis and modification of SO42−/TiO2, which directly influenced their catalytic activities.12,13 In this present work, composite oxide spinel ZnAl2O4 as a new superior system for synthesis of S2O82−/ZnAl2O4-x wt% Ce solid acid catalysts exhibited the characteristic advantages of the simple crystal structure and excellent structure stability in the process of synthesis and modification. Besides, S2O82−/ZnAl2O4-x wt% Ce solid acid catalysts had the advantages of easier preparation and lower cost compared to other type solid acid catalysts as shown in Table 3.
| Catalyst | Reaction conditions | Esterification efficiency (%) | Refs. | ||
|---|---|---|---|---|---|
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) na ratio na ratio |
Amountb (wt%) | Reaction time (h) | |||
| a The molar ratio of acetic acid to n-butanol.b Catalyst amount is calculated on the basis of total weight of the reactants. | |||||
| S2O82−/ZnAl2O4-4 wt% Ce | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1.55 | 3 | 95.9 | Present work |
| SO42−–TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 | 0.75 | 60.1 | 16 |
| SO42−–Ce0.02/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 | 0.75 | 97.1 | 16 |
| Mesoporous resin –SO3H | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.12 | 4 | 97.6 | 29 |
| Mesoporous carbon –SO3H | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.12 | 4 | 96.8 | 29 |
| [TPSPP]3PW12O40 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
5.38 | 1.5 | 97 | 30 |
| 20% TPA/AT-GMB | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.36 | 12 | 88 | 31 |
| Sulfate-promoted iron oxide | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.56 1.56 |
4.88 | 1 | 84.5 | 27 |
| 10 wt% MoO3/Al2O3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1 | 2 | 81 | 32 |
3.2 Catalytic characterization
In order to understand the crystal transformation process, Fig. 2 showed XRD pattern of ZnAl2O4-x wt% Ce (a) and S2O82−/ZnAl2O4-x wt% Ce solid acid catalyst (b). As shown in Fig. 2(a), the diffraction peaks at 2θ = 18.9°, 31.4°, 36.90°, 44.2°, 49.2°, 55.8°, 59.5° and 65.4° were observed in ZnAl2O4-x wt% Ce, which were the characteristic of ZnAl2O4 spinel phase (PDF no. 73-1961). Similar peaks were also observed in S2O82−/ZnAl2O4-x wt% Ce solid acid catalysts (shown in Fig. 2(b)), indicating that the carrier spinel of ZnAl2O4 had the prominent advantages of single crystal shape and stable structure in the process of modification and sulfation. However, there were also some differences in the samples. Compared with ZnAl2O4, ZnAl2O4-x wt% Ce (x = 2, 4, 6, 8, 10) displayed the new diffraction peaks of CeO2 because of cerium addition. Moreover, the characteristic peaks of CeO2 became stronger with the increase of Ce doping amount, indicating that Ce mainly existed in the form of CeO2. Besides, the weak diffraction peaks of ZnO phase were also observed in all samples of ZnAl2O4-x wt% Ce (x = 2, 4, 6, 8, 10), which might be related to the change of the chemical environment in the spinel lattice because of the cerium addition. ZnSO4·H2O and Al2(SO4)3 crystalline phases were observed in all samples of S2O82−/ZnAl2O4-x wt% Ce by comparison with ZnAl2O4-x wt% Ce. It might be attributed to the interaction between excess S2O82− and metal ions.33,34 A similar phenomenon was also observed in other SO42−/MxOy solid acid catalysts.35,36 In addition, the characteristics diffraction peaks of CeO2 became unapparent in all samples of S2O82−/ZnAl2O4-x wt% Ce, which might be of high dispersions or amorphous phases. In the meantime, the characteristic diffraction peaks of ZnO were not detected in all samples of S2O82−/ZnAl2O4-x wt% Ce, suggesting that Zn2+ might be in the form of ZnSO4·H2O.Fig. 3 recorded the FT-IR spectra of S2O82−/ZnAl2O4-x wt% Ce solid acid catalysts. Three strong bands around 670 cm−1, 557 cm−1 and 497 cm−1 were attributed to Al–O stretching vibrations, Zn–O stretching vibrations and Al–O bending vibrations, respectively, which belonged to spinel-type ZnAl2O4 structure.37 This result was in good agreement with the XRD results. The special bands in the range of 900–1400 cm−1 were detected in all samples and characterized the active acid structures of the catalysts.38 The specific bands in this region were attributed to the strong interaction between sulfuric groups and metal ions, which were correlated to their high catalytic activities. Among them, the bands at 975 cm−1, 1110 cm−1 and 1180 cm−1 corresponded to the symmetric and asymmetric stretching vibration of S–O bonds, respectively.39 The band at 1398 cm−1 was attributed to the symmetric and asymmetric stretching mode of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.40 The suction-induced complex S
O, respectively.40 The suction-induced complex S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O improved the electron-accepting capability of the metal atoms, which made the samples possess super acid sites.41 Besides, the band located at 1635 cm−1 was assigned to the deformation vibration of the adsorbed water.42 In order to evaluate the structural stability, Fig. 3 also showed the IR spectra of S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times. By contrast with the fresh catalyst, the typical bands at 670 cm−1, 557 cm−1 and 497 cm−1 were still observed in S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times, indicating that it had excellent structural stability. In particular, S2O82−/ZnAl2O4-4 wt% Ce showed similar intensity and characteristics bands in the range of 1400–900 cm−1 after being used repeatedly for six times. This result suggested that S2O82−/ZnAl2O4-4 wt% Ce also presented excellent resistance to the loss of the sulfur species, which might be one of the important reasons for its better reusability.
O improved the electron-accepting capability of the metal atoms, which made the samples possess super acid sites.41 Besides, the band located at 1635 cm−1 was assigned to the deformation vibration of the adsorbed water.42 In order to evaluate the structural stability, Fig. 3 also showed the IR spectra of S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times. By contrast with the fresh catalyst, the typical bands at 670 cm−1, 557 cm−1 and 497 cm−1 were still observed in S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times, indicating that it had excellent structural stability. In particular, S2O82−/ZnAl2O4-4 wt% Ce showed similar intensity and characteristics bands in the range of 1400–900 cm−1 after being used repeatedly for six times. This result suggested that S2O82−/ZnAl2O4-4 wt% Ce also presented excellent resistance to the loss of the sulfur species, which might be one of the important reasons for its better reusability.
 | ||
| Fig. 3 IR spectra of S2O82−/ZnAl2O4-x wt% Ce before reaction and S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times. | ||
To probe the element type and element valence state on the surface of the catalysts, XPS analysis for S2O82−/ZnAl2O4 and S2O82−/ZnAl2O4-4 wt% Ce was performed. It was found Zn atom maintained the same chemical valence states in two samples.43 Two peaks corresponding to Zn 2p3/2 and Zn 2p1/2 were all located at 1022.1 eV and 1045.1 eV in the two samples, respectively. However, the addition of Ce had a certain affect on the chemical valence states of Al atom, O atom and S atom. Accordingly, there were some significant differences in the XPS spectra of the two samples. Compared with S2O82−/ZnAl2O4, there were higher binding energy of Al 2p, O 1s and S 2p in the XPS spectra of S2O82−/ZnAl2O4-4 wt% Ce. The change of the electron binding energy was resulted from the different chemical environment of the atoms. So, we could suppose that the addition of Ce might affect the interaction between atoms and atom type in combination with each other. From the result of catalytic activity, it was apparently demonstrated that S2O82−/ZnAl2O4-4 wt% Ce had higher catalytic activity and better reusability than S2O82−/ZnAl2O4. XPS analysis in this section might be one of the possible explanations for this result. The peaks at 886.1 eV, 897.3 eV and 904.8 eV were corresponding to Ce 3d binding energy, demonstrating the cerium oxidation state was +4.44,45 As shown in Fig. 4, Ce was observed in the XPS spectra of S2O82−/ZnAl2O4-4 wt% Ce, indicating that Ce had been successfully introduced to the catalyst. With reference to the XRD result, the intensity of CeO2 diffraction peaks became not apparent in the sample. So, the detection of the Ce in XPS result further evidenced that CeO2 might be highly dispersed in the sample. In addition, it is noted that the peak corresponding to S 2p binding energy was clearly observed in the two samples, which was assigned to the sulfur oxidation state of +6.46 It is well known that S6+ plays a key role on the formation of the acidity structure. The suction-induced complex S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O promotes the electron-accepting ability for the metal atoms, making the samples possess supper acid. Accordingly, IR spectra of S2O82−/ZnAl2O4-4 wt% Ce showed the special bands in the range of 900–1400 cm−1, which were correlated to the active acid structures of the catalysts.
O promotes the electron-accepting ability for the metal atoms, making the samples possess supper acid. Accordingly, IR spectra of S2O82−/ZnAl2O4-4 wt% Ce showed the special bands in the range of 900–1400 cm−1, which were correlated to the active acid structures of the catalysts.
 | ||
| Fig. 4 XPS spectra of S2O82−/ZnAl2O4 and S2O82−/ZnAl2O4-4 wt% Ce. (1) S2O82−/ZnAl2O4; (2) S2O82−/ZnAl2O4-4 wt% Ce. | ||
In order to examine the thermo stability of the catalysts, the TG analysis was showed in Fig. 5. The TG curve exhibited three weight loss stages of the two samples. The first weight loss below 400 °C belonged to desorption of the physical adsorbed water. The second weight loss between 400 °C and 600 °C was assigned to the dehydroxylation or the removal of S2O82− from the surface of the catalysts. The third weight loss at a higher temperature range between 600 and 1000 °C was due to the gradual decomposition of the sulfur species on the surface of the catalysts.39 So, the third weight loss above 600 °C was closely related to the sulphur content and the acid sites on the surface of the samples. By contrast, the fresh S2O82−/ZnAl2O4-4 wt% Ce gave the mass weight loss of 15.89% above 600 °C. But, the fresh S2O82−/ZnAl2O4 had the relatively little weight loss of 8.03% above 600 °C. This result revealed that the addition of Ce was beneficial to improve the number of acid center and the acid strength, making the catalytic activity of S2O82−/ZnAl2O4-4 wt% Ce increase. This result was not only identified by NH3-TPD analysis, but also was in good consistent with the results of the catalytic activities. For the used catalysts, there was still some weight loss between 600 °C and 1000 °C, making the used catalysts still keep a certain catalytic activity. Comparing the used catalysts with the fresh catalysts, the weight loss between 600 °C and 1000 °C was significantly reduced. The weight loss of 15.89% in the fresh S2O82−/ZnAl2O4-4 wt% Ce was changed into the weight loss of 7.98% in the used S2O82−/ZnAl2O4-4 wt% Ce. For the used S2O82−/ZnAl2O4, the weight loss between 600 °C and 1000 °C was also reduced to 2.41%. The above results proved that the loss of sulfur species would inevitably occur in the process of acid catalytic reaction, which was the one of an important reasons to cause catalyst deactivation.29 This result was in good well with the result of the catalyst stability.
The NH3-TPD results was used to determine the acid strength distribution from the desorption temperature of NH3. The higher the desorption peak temperatures was, the stronger the acid strength was.40 Generally, the peaks below 450 °C were believed to be caused by the absorption of weak and moderate intensity. The second peaks between 450 °C and 650 °C were attributed to strong acidic sites. The last peaks above 650 °C belonged to super strong acidic sites.47 As shown in Fig. 6(a), all the samples showed the prominent broad desorption peaks in the range of 100 °C to 700 °C. Among them, the TPD profiles of S2O82−/ZnAl2O4 revealed two peaks of NH3 desorption, one was in the range of 100 °C to 450 °C and another one was between 450 °C and 650 °C, suggesting the presence of weak, moderate and strong acidic sites. Assuming that the peak area was proportional to the amount of the acid site, the intensity of weak and moderate acid sites was evident higher than that of strong acid sites in the case of S2O82−/ZnAl2O4 solid acid catalyst. The TPD profiles of S2O82−/ZnAl2O4-2 wt% Ce and S2O82−/ZnAl2O4-6 wt% Ce catalysts gave broad ammonia desorption peaks in the range of 450 °C and 650 °C with certain intensity, indicating the high concentration of strong acidic sites. The TPD profiles of S2O82−/ZnAl2O4-4 wt% Ce catalyst exhibited three distinct peaks of NH3 desorption, two were between 450 °C and 650 °C and another one was at above 650 °C. Specifically, the NH3 desorption peak above 650 °C was observed with high intensity, suggesting the high concentration of super acidic sites.48 Compared with S2O82−/ZnAl2O4, S2O82−/ZnAl2O4-x wt% Ce had the stronger acid strength. This result further demonstrated that the addition of Ce contributed to improve the number of acid center and the acid strength, which was consistent well with the results of the XPS and TG analysis. This could be the main reason for the higher catalytic activity of S2O82−/ZnAl2O4-x wt% Ce. Based on NH3-TPD results, it was clearly observed that the S2O82−/ZnAl2O4-4 wt% Ce catalyst exhibited the highest acid strength and had the maximum number of acidic sites.
 | ||
| Fig. 6 NH3-TPD spectra of fresh S2O82−/ZnAl2O4-x wt% Ce (x = 0, 2, 4, 6) (a) and used S2O82−/ZnAl2O4-4 wt% Ce (b). | ||
It is worthwhile to investigate the reasons for the deactivation of SO42−/MxOy solid acid catalysts. The above TG analysis speculated that the loss of sulfur species on the surface of S2O82−/ZnAl2O4-4 wt% Ce solid acid may be one of the reasons for its deactivation during the acid catalyzed reaction. In order to further explore the reason for the deactivation in the process of the reaction, Fig. 6(b) gave the TPD profiles of three samples, including fresh S2O82−/ZnAl2O4-4 wt% Ce, S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for the first time and six times. It was observed that the intensity of the peak above 650 °C become weaker with increasing the catalyst reuse times. Furthermore, it was also found that the amount of the acidic sites became less and less with the increase of the catalyst reuse times. After the catalyst being used repeatedly for six times, the peak above 650 °C was not detected and the amount of the acidic sites was decreased. Correspondingly, the catalytic activity was reduced. Combining the results of NH3-TPD with TG analysis, it might be concluded that S2O82−/ZnAl2O4-4 wt% Ce solid acid inevitable suffered from the loss of sulfur species, reducing the acid strength and decreasing the amount of the acidic sites. This was one of the essential reasons for the deactivation of S2O82−/ZnAl2O4-4 wt% Ce solid acid during the reaction. The result also had certain reference value for other SO42−/MxOy solid acid catalysts.
SEM images of fresh S2O82−/ZnAl2O4-4 wt% Ce and S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times were shown in Fig. 7. The fresh S2O82−/ZnAl2O4-4 wt% Ce displayed an aggregated-nanosheet appearance on the surface, which might be beneficial to the increase of the acid sites and the improvement of the catalytic activity.49 The catalyst after being used repeatedly for six times had slightly particles agglomeration. Combining with the experimental results of N2 adsorption analysis (as shown in Fig. 8), the N2 adsorption–desorption isotherms of both fresh and used catalysts showed the typical IV isotherm with the hysteresis loop in the low relative (P/P0) range of 0.4–1, indicating their representative mesoporous structures. Besides, the average pore size of fresh and used catalysts was 9.5 nm and 10.7 nm, respectively. In addition, Fig. 8 showed that the BET surface area of fresh S2O82−/ZnAl2O4-4 wt% Ce was 21.1 m2 g−1. Whereas, S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times was reduced to the 11.0 m2 g−1, which was probably due to the particles agglomeration as demonstrated by SEM images. The particles agglomeration and the decrease of the specific surface area might be the reasons for its decrease in the catalytic activity of S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times.
 | ||
| Fig. 7 SEM images of fresh S2O82−/ZnAl2O4-4 wt% Ce and S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times. | ||
 | ||
| Fig. 8 N2 adsorption–desorption isotherms and pore-size distributions of fresh S2O82−/ZnAl2O4-4 wt% Ce and S2O82−/ZnAl2O4-4 wt% Ce after being used repeatedly for six times. | ||
4. Conclusions
A new series of S2O82−/ZnAl2O4-x wt% Ce solid acid catalyst with stable spinel structures were successfully prepared by modifying S2O82−/ZnAl2O4 with Ce. The improved catalysis performance of Ce-modified S2O82−/ZnAl2O4 was attributed to the reasons that the addition of Ce improved the stability of the sulfur species and the acid strength of S2O82−/ZnAl2O4-x wt% Ce. Among them, the S2O82−/ZnAl2O4-4 wt% Ce catalyst performed the highest catalytic activity, which was ascribe to its highest acid strength and the maximum number of acidic sites. In addition, the optimum synthesis conditions over S2O82−/ZnAl2O4-4 wt% Ce solid acid catalyst were as follows: the molar ratio of acetic acid to n-butanol was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; catalyst amount was 1.55%; reaction time was 3 h. Under the optimum synthesis conditions, the esterification efficiency over S2O82−/ZnAl2O4-4 wt% Ce was up to 95.9%. Compared with unmodified S2O82−/ZnAl2O4, S2O82−/ZnAl2O4-4 wt% Ce solid acid catalyst showed the obviously better reusability, which might be attribute to its excellent structural stability and excellent resistance to the loss of the sulfur species. However, the loss of sulfur species would still inevitably occur in the process of acid catalytic reaction, which was one of an important reason to cause catalyst deactivation. At present, S2O82−/ZnAl2O4-x wt% Ce solid acid catalysts have not yet been extensively studied. The obtained results provided the possibility to prepare a large variety of solid acids based on composite oxide spinel.
3; catalyst amount was 1.55%; reaction time was 3 h. Under the optimum synthesis conditions, the esterification efficiency over S2O82−/ZnAl2O4-4 wt% Ce was up to 95.9%. Compared with unmodified S2O82−/ZnAl2O4, S2O82−/ZnAl2O4-4 wt% Ce solid acid catalyst showed the obviously better reusability, which might be attribute to its excellent structural stability and excellent resistance to the loss of the sulfur species. However, the loss of sulfur species would still inevitably occur in the process of acid catalytic reaction, which was one of an important reason to cause catalyst deactivation. At present, S2O82−/ZnAl2O4-x wt% Ce solid acid catalysts have not yet been extensively studied. The obtained results provided the possibility to prepare a large variety of solid acids based on composite oxide spinel.
Acknowledgements
The authors gratefully acknowledge the financial support from NSFC (21203170 and 41172051), ERCNGME (CUGNGM 20133), Self-DIRFC (2013–09) and NCSITP (201310491019 and 201510491034).Notes and references
- P. Barbaro, L. Gonsalvi, A. Guerriero and F. Liguori, Green Chem., 2012, 14, 3211–3219 RSC.
- J. M. Fraile, E. García-Bordejé and L. Roldán, J. Catal., 2012, 289, 73–79 CrossRef CAS.
- W. K. Yao, J. Li, Y. Feng, W. Wang, X. L. Zhang, Q. Chen, S. Komarneni and Y. J. Wang, RSC Adv., 2015, 5, 30485–30494 RSC.
- A. Corma, A. Martínez and C. Martínez, J. Catal., 1996, 164, 422–432 CrossRef CAS.
- E. L. S. Ngee, Y. J. Gao, X. Chen, T. M. Lee, Z. G. Hu, D. Zhao and N. Yan, Ind. Eng. Chem. Res., 2014, 53, 14225–14233 CrossRef CAS.
- S. Blagov, S. Parada, O. Bailer, P. Moritz, D. Lam, R. Weinand and H. Hasse, Chem. Eng. Sci., 2006, 61, 753–776 CrossRef CAS.
- F. C. Zheng, Q. W. Chen, L. Hu, N. Yan and X. K. Kong, Dalton Trans., 2014, 43, 1220–1227 RSC.
- Y. W. Peng, Z. G. Hu, Y. J. Gao, D. Q. Yuan, Z. X. Kang, Y. H. Qian, N. Yan and D. Zhao, ChemSusChem, 2015, 8, 3208–3212 CrossRef CAS PubMed.
- X. J. Shi, Y. L. Wu, P. P. Li, H. F. Yi, M. D. Yang and G. H. Wang, Carbohydr. Res., 2011, 346, 480–487 CrossRef CAS PubMed.
- F. Su and Y. H. Guo, Green Chem., 2014, 16, 2934–2957 RSC.
- A. Osatiashtiani, A. F. Lee, D. R. Brown, J. A. Melero, G. Moralese and K. Wilson, Catal. Sci. Technol., 2014, 4, 333–342 CAS.
- D. E. López, J. G. Goodwin, D. A. Bruce and E. Lotero, Appl. Catal., A, 2005, 295, 97–105 CrossRef.
- M. E. Sad, C. L. Padró and C. R. Apesteguía, Appl. Catal., A, 2014, 475, 305–313 CrossRef CAS.
- K. Takao, Catal. Today, 2003, 81, 57–63 CrossRef.
- K. Joseph Antony Raj, M. G. Prakash and B. Viswanathan, Catal. Sci. Technol., 2011, 1, 1182–1188 Search PubMed.
- G. Xiao, J. F. Zhou, X. Huang, X. P. Liao and B. Shi, RSC Adv., 2014, 4, 4010–4019 RSC.
- J. R. Sohn and D. C. Shin, Appl. Catal., B, 2008, 77, 386–394 CrossRef CAS.
- J. Y. Song, S. H. Chung, M. S. Kim, M. G. Seo, Y. H. Lee, K. Y. Lee and J. S. Kim, J. Mol. Catal. A: Chem., 2013, 370, 50–55 CrossRef CAS.
- L. Li, S. W. Liu, J. M. Xu, S. T. Yu, F. S. Liu, C. X. Xie, X. P. Ge and J. Y. Ren, J. Mol. Catal. A: Chem., 2013, 368–369, 24–30 CAS.
- G. D. Yadav and R. V. Sharma, J. Catal., 2014, 311, 121–128 CrossRef CAS.
- D. H. Guan, M. Q. Fan, J. Wang, Y. Zhang, Q. Liu and X. Y. Jing, Mater. Chem. Phys., 2010, 122, 278–283 CrossRef CAS.
- A. Q. Wang, X. L. Wu, J. X. Wang, H. Pan, X. Y. Tian and Y. L. Xing, RSC Adv., 2015, 5, 19652–19658 RSC.
- G. D. Fan, M. Shen, Z. Zhang and F. R. Jia, J. Rare Earths, 2009, 27, 437–442 CrossRef.
- H. Zhao, P. P. Jiang, Y. M. Dong, M. Huang and B. L. Liu, New J. Chem., 2014, 38, 4541–4548 RSC.
- T. F. Parangi, B. N. Wani and U. V. Chudasama, Ind. Eng. Chem. Res., 2013, 52, 8969–8977 CrossRef CAS.
- G. Kuriakose and N. Nagaraju, J. Mol. Catal. A: Chem., 2004, 223, 155–159 CrossRef CAS.
- W. P. Shi and J. W. Li, Catal. Lett., 2013, 143, 1285–1293 CrossRef CAS.
- J. R. Sohn, S. H. Lee and J. S. Lim, Catal. Today, 2006, 116, 143–150 CrossRef CAS.
- F. J. Liu, J. Sun, Q. Sun, L. F. Zhu, L. Wang, X. J. Meng, C. Z. Qi and F. S. Xiao, Catal. Today, 2012, 186, 115–120 CrossRef CAS.
- W. H. Zhang, Y. Leng, D. R. Zhu, Y. J. Wu and J. Wang, Catal. Commun., 2009, 11, 151–154 CrossRef CAS.
- S. K. Bhorodwaj and D. K. Dutta, Appl. Clay Sci., 2011, 53, 347–352 CrossRef CAS.
- G. Mitran, É. MakÓ, Á. Rédey and I. C. Marcu, Catal. Lett., 2010, 140, 32–37 CrossRef CAS.
- K. H. Jiang, D. M. Tong, J. Q. Tang, R. L. Song and C. W. Hu, Appl. Catal., A, 2010, 389, 46–51 CrossRef CAS.
- R. P. Yao, M. J. Zhang, J. Yang, D. L. Yi, J. Xu, F. Deng, Y. Yue and C. H. Ye, Acta Chim. Sin, 2005, 63, 269–273 CAS.
- H. P. Yan, Y. Yang, D. M. Tong, X. Xiang and C. W. Hu, Catal. Commun., 2009, 10, 1558–1563 CrossRef CAS.
- J. C. Martín, S. B. Rasmussen, S. Suárez, M. Yates, F. J. Gil-Llambías, M. Villarroel and P. Ávila, Appl. Catal., B, 2009, 91, 423–427 CrossRef.
- S. Farhadi and S. Panahandehjoo, Appl. Catal., A, 2010, 382, 293–302 CrossRef CAS.
- G. D. Yadav and B. A. Gawade, Catal. Today, 2013, 207, 145–152 CrossRef CAS.
- W. P. Shi, Catal. Lett., 2013, 143, 732–738 CrossRef CAS.
- P. F. Chen, M. X. Du, H. Lei, Y. Wang, G. L. Zhang, F. B. Zhang and X. B. Fan, Catal. Commun., 2012, 18, 47–50 CrossRef CAS.
- L. J. Liu, G. L. Zhang, L. Wang, T. Huang and L. Qin, Ind. Eng. Chem. Res., 2011, 50, 7219–7227 CrossRef CAS.
- Y. J. Wu, L. Qin, G. L. Zhang, L. Chen, X. W. Guo and M. Liu, Ind. Eng. Chem. Res., 2013, 52, 16698–16708 CrossRef CAS.
- L. Zhang, J. H. Yan, M. J. Zhou, Y. H. Yang and Y. N. Liu, Appl. Surf. Sci., 2013, 268, 237–245 CrossRef CAS.
- S. Lou, T. D. Nguyen-Phan, A. C. Johnston-Peck, L. Barrio, S. Sallis, D. A. Arena, S. Kundu, W. Q. Xu, L. F. J. Piper, E. A. Stach, D. E. Polyansky, E. Fujita, J. A. Rodriguez and S. D. Senanayake, J. Phys. Chem. C, 2015, 119, 2669–2679 Search PubMed.
- P. Sudarsanam, B. Mallesham, P. S. Reddy, D. Großmann, W. Grünert and B. M. Reddy, Appl. Catal., B, 2014, 144, 900–908 CrossRef CAS.
- C. W. Dunnill, Z. A. Aiken, A. Kafizas, J. Pratten, M. Wilson, D. J. Morgan and I. P. Parkin, J. Mater. Chem., 2009, 19, 8747–8754 RSC.
- H. Pan, J. X. Wang, L. Chen, G. H. Su, J. M. Cui, D. W. Meng and X. L. Wu, Catal. Commun., 2013, 35, 27–31 CrossRef CAS.
- C. C. Hwang and C. Y. Mou, Appl. Catal., A, 2009, 365, 173–179 CrossRef CAS.
- C. Tagusagawa, A. Takagaki, K. Takanabe, K. Ebitani, S. Hayashi and K. Domen, J. Catal., 2010, 270, 206–212 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |