Direct, rapid, solvent-free conversion of unactivated esters to amides using lithium hydroxide as a catalyst†
Shelli A. Millera and
Nicholas E. Leadbeater*ab
aDepartment of Chemistry, University of Connecticut, 55 North Eagleville Road, Storrs, CT 06269, USA. E-mail: nicholas.leadbeater@uconn.edu
bDepartment of Community Medicine & Health Care, University of Connecticut Health Center, The Exchange, 263 Farmington Ave, Farmington, CT 06030, USA
First published on 20th October 2015
Abstract
A simple, solvent-free methodology is reported for the direct conversion of esters to amides using lithium hydroxide as a catalyst. The approach allows for the preparation of a range of amide products as well as being applicable to the ring-opening of a representative lactone.
Introduction
Amides are one of the widest classes of compounds found in natural products as well as in pharmaceuticals.1 Their synthesis has been, and continues to be the focus of significant attention in synthetic chemistry.2,3 However, despite the wide array of methods available, the preparation of amides is often regarded as “expensive and inelegant”.4,5 The most common route involves the reaction of carboxylic acids with amines, but this often requires the use of stoichiometric quantities of activators. There have been concerted efforts made to develop catalytic approaches.6 One option involves the direct conversion of unactivated esters to amides. Indeed, this method is used in vivo by ribosomes for protein synthesis (amide bond formation).7 Esters are useful starting materials since they are common synthetic intermediates on the way to target compounds. Amidation of esters can be performed using a range of transition metal and lanthanide catalysts.8 Examples include the use of group (IV) metal alkoxide complexes (Fig. 1, eqn (1))9 and lanthanum trifluoromethanesulfonate (eqn (2)).10 Avoiding the use of transition metals, inorganic bases such as potassium phosphate (eqn (3))11 and sodium methoxide (eqn (4))12 have also been used as catalysts. These reactions hinge on the use of anhydrous conditions and, in the case of NaOMe, the exclusion of air. Both also take 20–120 h to reach completion. An organocatalyzed route has also been reported using a guanidine catalyst.13 There is also a report of the amidation of esters purportedly performed at 40 °C in the absence of solvent and in some cases the absence of catalyst.14,15As part of an ongoing project focused around transesterification reactions, we and others have found that when used in conjunction with calcium oxide, lithium salts prove to be highly efficient catalysts.16 We posited that this catalyst system may be useful for the conversion of esters to amides and hence embarked on a study to probe this. We report our results here.
Results and discussion
As a starting point we chose to use ethyl benzoate and benzylamine as test reagents, working with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the two. Performing the reaction solvent-free and using a 1
1 stoichiometry of the two. Performing the reaction solvent-free and using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of lithium nitrate and calcium oxide (5 mol% of each) as the catalyst, we obtained a trace of product after heating at 80 °C for 30 min (Table 1, entry 1). Using a microwave unit as an effective and safe way to operate at elevated temperatures, we performed the reaction at 200 °C for 15 min and obtained a 67% conversion (entry 2). By using a 1
1 mixture of lithium nitrate and calcium oxide (5 mol% of each) as the catalyst, we obtained a trace of product after heating at 80 °C for 30 min (Table 1, entry 1). Using a microwave unit as an effective and safe way to operate at elevated temperatures, we performed the reaction at 200 °C for 15 min and obtained a 67% conversion (entry 2). By using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 stoichiometric ratio of ester to amine we were able to increase the conversion to 78% (entry 3). We next performed two test reactions, one using just calcium hydroxide and one using lithium hydroxide, obtaining 8% and 74% conversions, respectively (entries 4 & 5). Using lithium nitrate as the catalyst also proved to be successful (entry 6), with the result being similar to that with lithium hydroxide, showing the importance of the lithium cation rather than the reaction simply being base-mediated.
1.5 stoichiometric ratio of ester to amine we were able to increase the conversion to 78% (entry 3). We next performed two test reactions, one using just calcium hydroxide and one using lithium hydroxide, obtaining 8% and 74% conversions, respectively (entries 4 & 5). Using lithium nitrate as the catalyst also proved to be successful (entry 6), with the result being similar to that with lithium hydroxide, showing the importance of the lithium cation rather than the reaction simply being base-mediated.
| Entry | Catalyst (mol%) | Ratio 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
Temp. (°C) | Time (min) | 3ab (%) |
|---|---|---|---|---|---|
| a Reactions performed using 5 mmol of 1a.b Values in parentheses indicate isolated yields, all other values are percent conversions by 1H-NMR spectroscopy. | |||||
| 1 | LiNO3 (5), CaO (5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 30 | 4 |
| 2 | LiNO3 (5), CaO (5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
200 | 15 | 67 |
| 3 | LiNO3 (5), CaO (5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
200 | 15 | 78 |
| 4 | Ca(OH)2 (5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
200 | 15 | 8 |
| 5 | LiOH (5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
200 | 15 | 74 |
| 6 | LiNO3 (5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
200 | 15 | 79 |
| 7 | LiOH (5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
200 | 30 | 80 |
| 8 | LiOH (5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
200 | 60 | 87 |
| 9 | LiOH (5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
200 | 30 | 84 |
| 10 | LiOH (7.5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
200 | 30 | 88 (78) |
Deciding to focus our attention on lithium hydroxide as the catalyst, we next probed the effects of time and catalyst loading on the reaction. Extending the reaction time to 60 min improved the product conversion to 87% (entries 7 & 8). Increasing the stoichiometric ratio of ester to amine from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 to 1
1.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 had a negligible effect on the reaction (entry 9). However, by working at the lower substrate ratio but increasing the catalyst loading to 7.5 mol% an 88% product conversion could be obtained in just 30 min (entry 10). Thus, optimal reaction conditions involved heating a 1
2 had a negligible effect on the reaction (entry 9). However, by working at the lower substrate ratio but increasing the catalyst loading to 7.5 mol% an 88% product conversion could be obtained in just 30 min (entry 10). Thus, optimal reaction conditions involved heating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 stoichiometric ratio of ester to amine at 200 °C for 30 min using 7.5 mol% LiOH as the catalyst.
1.5 stoichiometric ratio of ester to amine at 200 °C for 30 min using 7.5 mol% LiOH as the catalyst.
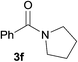
With optimized conditions in hand, we next explored the substrate scope of the reaction. Using ethyl benzoate as the ester component, we first screened a number of amine coupling partners (Table 2). The reaction was compatible with primary and secondary amines (Table 2, entries 1–6). However, employing aniline and cyclohexylamine did not prove to be successful, presumably for electronic and steric reasons respectively (entries 7 & 8).
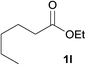
We next screened the reaction between benzylamine and a number of esters (Table 3). Amides were readily prepared from ethyl benzoates bearing electron donating and withdrawing groups as well as phenyl benzoate and an aliphatic ester (Table 3, entries 1–5). The exception was ethyl 4-nitrobenzoate, 1m, where polymerization occurred at the elevated temperature used (entry 6).
While effective and rapid, one potential drawback of our methodology is that the high temperature at which the reaction is performed could lead to the decomposition of more delicate substrates. We therefore wanted to benchmark our methodology against the literature report of a solvent and catalyst free procedure for the amidation of N-protected amino acid esters at 40 °C.14,15 The reaction involves heating the protected amino acid ester and amine at 40 °C for 20 min–36 h. We first tried using these conditions for the amidation of ethyl benzoate with benzylamine. No product was obtained after stirring at 40 °C for 24 h. We next attempted to reproduce the reported amidation of the benzyl ester of N-benzoylglycine, 1n, with benzylamine, but the reaction was not successful in our hands. However, by employing our reaction conditions but operating at 90 °C for 30 min, we were able to obtain a >95% conversion of 1n to the benzamide analogue 3n (Scheme 1). This hints at the fact that with more delicate substrates our methodology could be employed by working at lower temperatures.
Finally, we were interested in applying our reaction conditions to the ring-opening of a lactone. This was indeed possible. Working on the 5 mmol scale with δ-valerolactone (1o) and benzylamine as substrates, 3o was obtained in 87% yield (Scheme 2). To show the scalability of this reaction and the methodology in general, we performed the ring-opening on a 30 mmol scale and obtained an identical isolated product yield.
Conclusions
In summary, we present a methodology for the direct conversion of esters to amides using lithium hydroxide as a catalyst. The approach allows for the preparation of a range of amide products as well as being applicable to the ring-opening of a representative lactone.Experimental section
General considerations
Reactions were performed using a CEM Discover microwave unit. NMR Spectra (1H, 13C) were performed at 298 K on either a Brüker Avance Ultra Shield 300 MHz NMR, Brüker DRX-400 400 MHz NMR, or Brüker Avance 500 MHz NMR. 1H-NMR spectra obtained in CDCl3 were referenced to residual non-deuterated chloroform (7.26 ppm) in the deuterated solvent. 13C-NMR spectra obtained in CDCl3 were referenced to chloroform (77.3 ppm). Flash chromatography and silica plugs utilized Dynamic Adsorbents Inc. Flash Silica Gel (60 Å porosity, 32–63 μm).Chemicals
Deuterated chloroform was purchased from Cambridge Isotope Laboratories and stored over 4 Å molecular sieves. Ethyl benzoate, benzylamine, pyrrolidine, and cyclohexylamine were purchased from Sigma-Aldrich. Lithium hydroxide and 1,2,3,4-tetrahydroisoquinoline were purchased from Acros Organic. Ethyl-p-nitrobenzoate, ethyl-4-bromobenzoate, n-octylamine, and δ-valerolactone were purchased from Alfa Aesar. Aniline was purchased from Fisher Scientific. Ethyl-4-(trifluoromethyl)benzoate and 4-methoxybenzylamine were purchased from Oakwood Chemicals. Ethyl hexanoate and benzyl benzoate were purchased from Chem Service.Representative experimental procedure: the preparation of N-benzyl benzamide (3a)
To a 10 mL capacity glass microwave tube equipped with a stir bar was added ethyl benzoate (0.75 g, 5 mmol, 1 equiv.), benzylamine (0.80 g, 7.5 mmol, 1.5 equiv.), and lithium hydroxide (0.009 g, 0.375 mmol, 0.075 equiv.). The tube was sealed with a septum and placed into the microwave cavity. The reaction mixture was heated to 200 °C using an initial microwave power of 200 W and setting a pressure cut-off of 250 psi for safety purposes. Once at temperature, the contents of the tube were maintained at 200 °C for 30 min. After completion of the heating time, the reaction vessel was cooled to 50 °C before removing from the microwave unit. Product conversion was then determined by 1H NMR. To isolate the product, the contents of the tube were triturated with hexanes, filtered through a fritted funnel, and washed with more hexanes. The solid precipitate was dissolved in EtOAc and filtered through a pad of Celite. The ethyl acetate was removed in vacuo by rotary evaporation to afford the pure amide product, N-benzyl benzamide as a white solid (0.823 g, 78%).1H NMR (400 MHz, CDCl3) δ ppm 4.56 (d, J = 5.84 Hz, 2H) 7.26–7.32 (m, 5H) 7.35 (t, J = 7.59 Hz, 2H) 7.46 (t, J = 1.00 Hz, 1H) 7.81 (d, J = 7.79 Hz, 2H). 13C NMR (CDCl3, 100 MHz) δ ppm 44.01 (CH2) 127.24 (CH) 127.45 (CH) 127.82 (CH) 128.56 (CH) 128.72 (CH) 131.52 (CH) 134.48 (C) 138.56 (C) 167.73 (C).
Acknowledgements
This work was supported by the National Science Foundation (CAREER Award CHE-0847262).Notes and references
- For background, see: A. Greenberg, C. M. Breneman and J. F. Liebman, The Amide Linkage: Selected Structural Aspects in Chemistry, Biochemistry, and Materials Science, Wiley, New York, 2000 Search PubMed.
- For background, see: M. B. Smith, Compendium of Organic Synthetic Methods, vol. 9, ch. 6, Wiley, New York, 2000 Search PubMed.
- For recent reviews, see: (a) S. C. Stolze and M. Kaiser, Synthesis, 2012, 44, 1755 CrossRef; (b) A. El-Faham and F. Albericio, Chem. Rev., 2011, 111, 6557 CrossRef CAS PubMed.
- V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471 CrossRef CAS PubMed.
- For a green chemistry perspective, see: D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. J. L. Leazer, R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411 RSC.
- R. M. Lanigan and T. D. Sheppard, Eur. J. Org. Chem., 2013, 7453 CrossRef CAS.
- (a) J. L. Hansen, T. M. Schmeing, P. B. Moore and T. Steitz, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 11670 CrossRef CAS PubMed; (b) M. Beringer and M. V. Rodnina, Mol. Cell, 2007, 26, 311 CrossRef CAS PubMed; (c) K. Watanabe, Y. Toh, K. Suto, Y. Shimizu, N. Oka, T. Wada and K. Tomita, Nature, 2007, 449, 867 CrossRef CAS PubMed.
- C. L. Allen and J. M. J. Williams, Chem. Soc. Rev., 2011, 40, 3405 RSC.
- C. Han, J. P. Lee, E. Lobkovsky and J. A. Porco, J. Am. Chem. Soc., 2005, 127, 10039 CrossRef CAS PubMed.
- H. Morimoto, R. Fujiwara, Y. Shimizu, K. Morisaki and T. Ohshima, Org. Lett., 2014, 16, 2018 CrossRef CAS PubMed.
- N. Caldwell, C. Jamieson, I. Simpson and A. J. B. Watson, ACS Sustainable Chem. Eng., 2013, 1, 1339 CrossRef CAS.
- T. Ohshima, Y. Hayashi, K. Agura, Y. Fujii, A. Yoshiyama and K. Mashima, Chem. Commun., 2012, 48, 5434 RSC.
- C. Sabot, K. A. Kumar, S. Meunier and C. Mioskowski, Tetrahedron Lett., 2007, 48, 3863 CrossRef CAS.
- K. C. Nadimpally, K. Thalluri, N. B. Palakurthy, A. Saha and B. Mandal, Tetrahedron Lett., 2011, 52, 2579 CrossRef CAS.
- K. Thalluri, K. C. Nadimpally, A. Paula and B. Mandal, RSC Adv., 2012, 2, 6838 RSC.
- D. Kumar and A. Ali, Energy Fuels, 2010, 24, 2091 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C-NMR spectral data and copies of the spectra. See DOI: 10.1039/c5ra21394k |
| This journal is © The Royal Society of Chemistry 2015 |