Hydroconversion of carboxylic acids using mesoporous SBA-15 supported NiMo sulfide catalysts under microwaves
Titiya Meechaiab,
Emmanuel Leclercb,
Dorothée Laurentib,
Ekasith Somsooka and
Christophe Geantet*b
aNANOCAST Laboratory, Center for Catalysis, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahidol University, 272 Rama VI Rd., Thung Phaya Thai, Ratchathewi, Bangkok 10400, Thailand
bIRCELYON UMR 5256 CNRS Université Lyon 1, 2 Av A Einstein 69626, Villeurbanne Cedex, France. E-mail: christophe.geantet@ircelyon.univ-lyon1.fr
First published on 10th November 2015
Abstract
Hydrogenation of octanoic acid was performed in a continuous manner, using microwaves (MW), and a supported metal sulfide catalyst. SBA-15, AlSBA-15 and ZrSBA-15 supported NiMo hydrotreating catalysts were prepared by an incipient wetness impregnation method in order to investigate the role of support acidity. Extrudates of the supported NiMo powders were prepared and sulfided. Octanoic acid in dodecane (10%) was introduced in the continuous flow reactor by means of an HPLC pump and co-fed with hydrogen at a working pressure of 0.5 MPa, while varying the reaction parameters such as temperature and feed flow rate (0.1, 0.25, and 0.5 mL min−1). The power applied to the monomodal cavity varied in the range of 15–150 W and corresponding temperature from 200–350 °C. Catalysts and supports were characterized by small- and wide-angle XRD, N2-physisorption (BET, BJH), HRTEM, ICP-MS, and NH3-TPD. The thermal response under MW showed that the extrudates containing SBA-15 (with or without Al or Zr) exhibited a strong MW response. The comparison of the catalytic activities showed that all SBA-15 supported NiMo catalysts exhibited the same activity range but the selectivity as compared to NiMo/Al2O3 catalysts was different.
1. Introduction
Despite being an exhaustible fossil resource, crude oil currently remains the main source of transportation fuels. One alternative is provided by biofuels and more specifically for diesel by the transesterification of vegetable oils. However, biodiesel has some drawbacks such as a limited content in conventional gas oil, a smaller heating value etc. For these reasons, biodiesel is not fully compatible with existing diesel engines and it is commercialized as a mixture with conventional diesel.1,2 In order to circumvent these drawbacks, vegetable oil can be hydrotreated (so called green diesel) to eliminate the oxygen and yield a renewable fuel with features similar to conventional diesel.3–5 Hydrodeoxygenation (HDO) of triglycerides leads first to the formation of carboxylic acids. Their conversion proceeds on conventional hydrotreating catalysts by three general routes that consist of ketonization, hydrogenolysis and decomposition (decarboxylation and decarbonylation). On sulfide catalysts, hydrogenation steps have been clearly identified with the help of theoretical modelling but the decomposition routes remains unclear.6,7 In the field of HDT catalysis, support effect are known to affect performances, dispersion, active phase and reaction pathways.8 Among alternative supports to conventional alumina, SBA 15 mesostructured support exhibit large pores and its acidity can offer the possibility to tailor the acidity by introducing substituting elements. Therefore, SBA-15 has been studied as support for NiMo hydrotreating catalysts by various researchers.9–13 The important modification included the incorporation of heteroatoms such as Ti, Zr, and Al in the framework of SBA-15,14,15 modifying acidity, texture, and dielectric properties. Hydrotreating properties of such modified SBA-15 have been also investigated.16–20 It is generally considered that by improving the acidity, hydrotreating performances are improved. The weak interaction between silica and molybdenum results in lower dispersion and higher stacking of the lamellar active phase, which in some cases may benefit to catalytic activity.21 Hence, in the present study, we investigated the hydrotreating of octanoic acid, under MW, in a fixed-bed reactor using NiMo on SBA-15, ZrSBA-15, and AlSBA-15 catalysts. Whereas the internal temperature distribution of a material subject to conventional heating is limited by its thermal conductivity, with MW, the heating is introduced into the sample from the liquid to the surface of the catalysts. In contrast, microwave heating results in each element of the material being heated individually and selectively, in our case the catalysts itself is heated selectively.22 The mesoporous properties of SBA15 could be helpful for trapping the reactant closer to the catalyst. Therefore, microwaves heating brings a larger temperature gradient between catalyst and surrounding species leading to acceleration of desorption and species transport in the system.23–252. Material and methods
2.1 Materials experimental
P123 (EO20PO70EO20, BASF, Mw = 5800), TEOS (tetraethyl orthosilicate), aluminum iso-propoxide, zirconyl chloride, boehmite, ammonium heptamolybdate, nickel nitrate, octanoic acid, dodecane, hexadecane were purchased from Sigma Aldrich and used without further purification.2.2 Support preparation
The SBA-15 was synthesized with 4 g of P123 that was dissolved in the solution of deionized water and 4 mol L−1 of HCl. It was stirred at 40 °C for 2 h and then 8.5 g of TEOS was slowly added and stirred at 40 °C for 20 h. The gel mixture was transferred into a Teflon bottle and aged at 100 °C for 24 h. After that the sample was calcined at 550 °C for 6 h.19,26–28Compositions of the catalysts atre reported in Table 1. The Zr-SBA-15 was prepared in the absence of HCl and using ZrOCl2·8H2O as zirconium precursor with the same procedure used for SBA-15, but was aged at 100 °C for 2 days.29–31
| Catalysts | Ni (% wt) | Mo (% wt) | Zr or Al (% wt) |
|---|---|---|---|
| NiMo/SBA-15 | 2.54 | 13.49 | |
| Ex-NiMo/SBA-15 | 0.75 | 4.10 | |
| NiMo/Zr-SBA-15 | 2.11 | 11.35 | Zr = 6.4 |
| Ex-NiMo/Zr-SBA-15 | 0.71 | 3.94 | |
| NiMo/Al-SBA-15 | 1.99 | 10.79 | Al = 0.16 |
| Ex-NiMo/Al-SBA-15 | 0.73 | 4.09 | |
| NiMo/Al2O3 | 3.00 | 14.00 |
To get Al-SBA-15, P123 was added to 30 mL of water. After stirring for few hours, a clear solution was obtained. Thereafter, aluminium iso-propoxide and the required amount of HCl (pH = 1.5) were added, and the solution was stirred at 40 °C for another 2 h. Then, 8.5 g of TEOS was added dropwise with vigorous stirring at 40 °C for 24 h to get a gel and finally crystallized in a Teflon-lined autoclave at 100 °C for 2 days. The crystals were filtered off, dried and calcined in air at 550 °C for 6 h.32,33
2.3 Catalyst preparation
All the catalysts were prepared by standard incipient wetness impregnation (IWI) technique. Molybdenum and nickel precursor salts solutions, with appropriate concentration, were impregnated on the supports. After each impregnation, the catalysts were dried at room temperature for 24 h and at 100 °C for 24 h and then calcined at 500 °C for 5 h.9,11,17,18,30,31,34,35Extrudates were obtained by mixing 3 g of boehmite AlOOH (binder) with 1 g of each catalyst, and nitric acid. This mixture was carefully mixed and then extruded with a syringe, dried and calcined under air at 500 °C for 2 h. The nomenclature Ex- will be used for these various extrudates.
Al content was kept low in order to promote slightly the acidity, since higher loading (Si/Al 5 or 10) were found to induce a severe cracking in the conversion methyl stearate on NiMo supported AlSBA-15 catalysts.36
Ammonia TPD measurements were achieved with BELCAT-M apparatus (BEL JAPAN, INC.). 100–200 mg of catalysts were pretreated at the calcination temperature for 1 h under He flow (50 mL min−1, NTP). After cooling down to 100 °C, NH3 was absorbed by flowing the catalysts under 5% NH3 in He for 30 min (30 mL min−1, NTP) followed by He desorption treatment at 100 °C for 15 min to remove physisorbed molecules. The catalysts were then heated under He flow (50 mL min−1, NTP) up to 450 °C with a heating rate of 8 °C min−1. Powder X-ray diffraction patterns were achieved on a Bruker D8 Advance A25 diffractometer equipped with a Ni filter (Cu K radiation: 0.154184 nm) and a one-dimensional multistrip detector (Lynxeye, 192 channels on 2.95°). Transmission electron microscopy (TEM) was performed using a JEOL 2010 microscope equipped with an EDS LINK-ISIS detector. The accelerating voltage was 200 kV with LaB6 emission current, a point to point resolution of 0.19 nm. A few mg of the samples, ultrasonically dispersed in an ethanol suspension, were deposited on a holey carbon copper grid.
2.4 Hydrodeoxygenation (HDO) in fixed-bed reactor under single-mode MW
A monomodal cavity has been designed to perform HDO reaction, under H2 pressure, in a continuous manner. The apparatus consists of a generator (2 kW, 2.45 GHz Muegge magnetron), circulators, a Tristan automatic tuning system (Muegge, HOMERHM software) linked to an adapted waveguide resonant cavity equipped with an iris (I) and plunger (P) close to the system described by Siores et al. or Suttisawat et al.37 Temperature was measured with a pyrometer focused on the reactor (Raytek). The cylindrical volume of the quartz reactor interacting with the MW in the optimized position of I and P for getting the maximum energy in the reactor filled with 3 g of sulfided NiMo/SBA-15 hydrotreating catalyst extrudates deposited on a quartz frit (volume 24 cm3). Response to MW excitation of the supports oxide catalysts and sulfide catalysts was investigated by 10 watt increments of input energy after stabilization of the temperature. Octanoic acid (OA) in dodecane (10%) was introduced in the continuous flow reactor by the mean of an HPLC pump and co-feeded with hydrogen under 0.5 MPa of pressure. Power applied to the cavity varies in the range of 15–150 W and corresponding temperature from 200–350 °C depending on the nature of the solid. Various flow rates (0.1, 0.25, and 0.5 mL min−1) were also used. Under these conditions, the octanoic acid was converted and reactant and hydrogenation products were analyzed by GC-FID (HP 5890, CPSil 5 column 50 m, 0.32 mm, 5 μm) (Scheme 1).3. Results and discussion
3.1 Thermal response of supports and catalysts
The thermal response of the catalytic solids to MW irradiation is given in Fig. 1. The benefit of a monomodal cavity is to concentrate the energy on the catalyst in the reactor section localized in the cavity. These preliminary experiments are also required in order to check if any thermal “runaway” cannot be reached under reaction conditions. The supports alone and catalysts under their oxide and sulfide form were first investigated in powder form. The thermal response is linked to electric permittivity, with ε′ which characterizes the ability to propagate microwaves into the material and ε′′ (imaginary part) which reflects the ability of material to dissipate the energy. The loss tangent tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ defined as the ratio of these two parameters is also commonly used to describe the dielectric losses. Silica based solids are known as poor absorbers, alumina slightly better (tan
δ defined as the ratio of these two parameters is also commonly used to describe the dielectric losses. Silica based solids are known as poor absorbers, alumina slightly better (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 0.066)38 but MoS2 exhibits good adsorption properties (tan
δ = 0.066)38 but MoS2 exhibits good adsorption properties (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 0.192). In addition dispersion of MoS2 on alumina enhances dielectric properties and dielectric loss tangent (or dissipation factor by a factor higher than 10).39 This trend is reflected in Fig. 1, the dispersion of molybdenum based sulfides at the surface of the catalyst enhances thermal response. This clearly suggests that energy will be preferentially transferred to the active phase.
δ = 0.192). In addition dispersion of MoS2 on alumina enhances dielectric properties and dielectric loss tangent (or dissipation factor by a factor higher than 10).39 This trend is reflected in Fig. 1, the dispersion of molybdenum based sulfides at the surface of the catalyst enhances thermal response. This clearly suggests that energy will be preferentially transferred to the active phase.
 | ||
| Fig. 1 Thermal response of MW irradiation in function of the power applied for different supports, oxides and sulfide catalysts. | ||
Concerning the dielectric properties of the extrudates (with about 3 times more alumina than silica based catalyst), the thermal response of the mixture of alumina and catalysts provide a strong response as illustrated by Fig. 2. It can be seen that small power inputs on the catalytic extrudates allow reaching easily temperatures in the required range of 200–350 °C to proceed to HDO. Due to the relatively low loading incorporated, hetero-atoms (Al, Zr) in the SBA-15 do not drastically modify the dielectric response of the extrudates but all the samples exhibit an almost similar answer to MW excitation, closer than sulfided NiMo/Al2O3 one.
The reactant and solvent themselves exhibit a poor response to microwave excitation at this power range; therefore during the catalytic reaction a temperature gradient occurs in the reactor between the hot surface catalysts and colder reaction mixture.
3.2 Characterizations of supports and catalysts
The textural properties of the catalysts and extrudates are given in Table 2. Mesoporous silica exhibit high specific surface are as strongly reduced by the introduction of the active phase by pore plugging. A narrow distribution of pores with an average porous diameter varying in the range of 5–9 nm is observed for the mesoporous samples and another contribution due to alumina centered at 3.5 nm is observed on the extrudates. Finally, the BET surface area, the pore volume and the average pore diameter of the three extrudates catalysts are very similar.| Catalysts | Surface area, SBET (m2 g−1) | Pore volume, Vp (cm3 g−1) | Average pore diameter, Dp (nm) |
|---|---|---|---|
| SBA-15 | 958 | 1.34 | 5.6 |
| NiMo/SBA-15 | 434 | 0.85 | 7.9 |
| Ex-NiMo/SBA-15 | 337 | 0.52 | 6.2 |
| Zr-SBA-15 | 728 | 174 | 9.3 |
| NiMo/Zr-SBA-15 | 448 | 1.1 | 8.8 |
| Ex-NiMo/Zr-SBA-15 | 339 | 0.5 | 6.3 |
| Al-SBA-15 | 1098 | 1.26 | 5.7 |
| NiMo/Al-SBA-15 | 508 | 0.72 | 5.7 |
| Ex-NiMo/Al-SBA-15 | 343 | 0.49 | 5.5 |
| NiMo/Al2O3 | 146 | 0.48 | 2.5 |
XRD patterns of SBA15 samples NiMo/SBA-15, NiMo/ZrSBA-15, NiMo/AlSBA-15 oxide samples exhibit respectively d100 spacing of 9.5, 11 and 10.1 nm characteristic of the organized mesoporous structure (Fig. 4).27,40,41 Wide-angle XRD evidences the presence of crystalline MoO3 (black dots in Fig. 5) indicative of the weak interaction of Mo precursors with silica based supports.30,35,42,43 The active phase of the catalysts is based on lamellar nanocrystallites of MoS2 as illustrated by Fig. 3. The distribution of stacking degree and slab length, metal dispersion follows the order ExNiMo/AlSBA-15 > ExNiMo/ZrSBA-15 > ExNiMo/SBA-15 (see Fig. 3). A higher number of MoS2 stacks (average number 6) and a long average slab length (7–17 nm) indicate a poorer dispersion as compared to conventional HDT catalysts.31
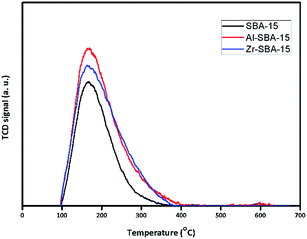
The acidic properties of the oxidic catalysts were evaluated by ammonia TPD, background corrected and normalized per g of solid spectrum are presented in Fig. 6. The amount of ammonia adsorbed on the surface of NiMoS/SBA-15, NiMoS/ZrSBA-15, NiMoS/AlSBA-15 samples were respectively 258, 394 and 413 μmol g−1 evidencing that the doping of SBA enhances the amount of acidic sites, even with a low ratio for Al, as it was already reported in the literature.34,36,44
3.3 Catalytic properties
Catalytic properties were investigated by varying reaction temperature, contact time and octanoic acid (OA) concentration. The kinetic order with respect to OA was determined by fitting the kinetic order from the following equation:with LHSV in h−1 and OA input and output concentrations. n was found to be in the range of 2.5–3 (see Fig. 7) indicating complex mechanisms and a strong disparity in the rate constants of the different reaction pathways (DCO versus HDO). At 330 °C, all the catalysts have almost the same apparent rate constant close to kapp: 1 mmol2 g−2 h−1 except Ex-NiMoS/AlSBA-15 which is two times less active as it can be seen on Table 3.
 | ||
| Fig. 7 Kinetic order determination at different LHSV for ExNiMoS/SBA-15, ExNiMoS/ZrSBA-15, ExNiMoS/AlSBA-15 and ExNiMoS/Al2O3 catalysts at 350 °C. | ||
| Catalysts | Rate constants: kapp (1 mmol2 g−2 h−1) | |
|---|---|---|
| 330 °C | 350 °C | |
| Ex-NiMo/SBA-15 | 1.1 | 1.8 |
| Ex-NiMo/Zr-SBA-15 | 1.3 | 2.5 |
| Ex-NiMo/Al-SBA-15 | 0.2 | 1.2 |
| Ex-NiMo/Al2O3 | 1.4 | 3.2 |
In fact, the hydroconversion of OA proceeds via two main well-known pathways: the HDO route which lead to C8 deoxygenated products via hydrogenation/hydrogenolysis reactions, and the DCO pathway which involves decarboxylation/decarbonylation reaction and lead to C7 deoxygenation products. Intermediates like octanal, octanol are also observed as well as esterification products like octyloctanoate (Scheme 2).
 | ||
| Scheme 2 Reaction pathways of octanoic acid (OA) deoxygenation showing the products of DCO and HDO pathways. | ||
The use of different supports may provide improved activities or different selectivities. Fig. 8 illustrates the selectivity observed with the different catalysts at a close level of conversion. The occurrence of the HDO pathway compared to DCO (decarboxylation/decarbonylation) route can be seen by looking at the C8/C7 ratios which include only alkenes and alkanes. The C8/C7 ratio is close to 0.4 for all based SBA-15 catalysts whereas it reached 1.6 for the NiMo on alumina catalyst. This result suggests that the NiMo catalyst supported on alumina favored the HDO pathway and on the contrary the NiMo catalysts supported on SBA-15, modified or not by Al or Zr, lead to the DCO route. In addition, Al containing SBA-15 supported NiMo exhibited a higher yield of octyloctanoate coming from esterification between octanol and OA, compared to the other catalysts, suggesting a contribution of stronger acid sites responsible of the undesired condensation reaction.
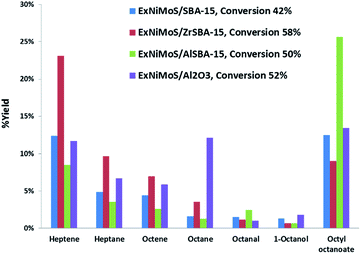 | ||
| Fig. 8 Products yields obtained for OA HDO over supported NiMo hydrotreating catalysts at 350 °C (catalyst = 3 g, flow rate = 0.25 mL min−1, P = 0.5 MPa, and [OA]o = 0.7 mmol g−1). | ||
4. Conclusions
This work illustrates the possibility to use MW activation under continuous flow and pressure conditions to convert octanoic acid, a model of the main intermediate of triglyceride hydrogenation. A low energy input (# 50 W) enables to carry out the conversion in temperature range similar to those used in industrial thermal processes. Even if the dispersion of sulfide catalysts is worth than the one obtained on alumina, by using SBA-15, the catalytic activities remain close to that of a reference catalyst. SBA-15 based catalyst provided a favored route with respect to decarboxylation as compared to NiMo on alumina catalyst. However, a higher acidity obtained by Al substitution in SBA-15 is unfavorable since it promotes the esterification route.Acknowledgements
The financial supports by Center of Excellence for Innovation in Chemistry (PERCH-CIC), Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0094/2552) to the PhD student 3.II.MU./52/AN.1, and the Institute of Researches on Catalysis and Environment in Lyon (IRCELYON) are acknowledged. J. F. Rochas from CETIAT and Total are acknowledged for their help and support for setting MW reactor.References
- J. Mikulec, J. Cvengroš, Ľ. Joríková, M. Banič and A. Kleinová, J. Cleaner Prod., 2010, 18, 917–926 CrossRef CAS.
- J. C. Serrano-Ruiz, E. V. Ramos-Fernandez and A. Sepulveda-Escribano, Energy Environ. Sci., 2012, 5, 5638–5652 CAS.
- M. Snåre, I. Kubičková, P. Mäki-Arvela, K. Eränen and D. Y. Murzin, Ind. Eng. Chem. Res., 2006, 45, 5708–5715 CrossRef.
- P. Šimáček, D. Kubička, G. Šebor and M. Pospíšil, Fuel, 2010, 89, 611–615 CrossRef.
- G. W. Huber and A. Corma, Angew. Chem., Int. Ed., 2007, 46, 7184–7201 CrossRef CAS PubMed.
- C. Dupont, R. Lemeur, A. Daudin and P. Raybaud, J. Catal., 2011, 279, 276–286 CrossRef CAS.
- M. Ruinart de Brimont, C. Dupont, A. Daudin, C. Geantet and P. Raybaud, J. Catal., 2012, 286, 153–164 CrossRef CAS.
- M. Breysse, P. Afanasiev, C. Geantet and M. Vrinat, Catal. Today, 2003, 86, 5–16 CrossRef CAS.
- S. Badoga, K. C. Mouli, K. K. Soni, A. K. Dalai and J. Adjaye, Appl. Catal., B, 2012, 125, 67–84 CrossRef CAS.
- G. M. Dhar, G. M. Kumaran, M. Kumar, K. S. Rawat, L. D. Sharma, B. D. Raju and K. S. R. Rao, Catal. Today, 2005, 99, 309–314 CrossRef CAS.
- P. Rayo, M. S. Rana, J. Ramírez, J. Ancheyta and A. Aguilar-Elguézabal, Catal. Today, 2008, 130, 283–291 CrossRef CAS.
- P. Rayo, J. Ramírez, M. S. Rana, J. Ancheyta and A. Aguilar-Elguézabal, Ind. Eng. Chem. Res., 2008, 48, 1242–1248 CrossRef.
- K. Soni, K. C. Mouli, A. K. Dalai and J. Adjaye, Catal. Lett., 2010, 136, 116–125 CrossRef CAS.
- T. Klimova, L. Peña, L. Lizama, C. Salcedo and O. Y. Gutiérrez, Ind. Eng. Chem. Res., 2008, 48, 1126–1133 CrossRef.
- L. Lizama and T. Klimova, J. Mater. Sci., 2009, 44, 6617–6628 CrossRef CAS.
- A. Romero-Pérez, A. Infantes-Molina, E. Rodríguez-Castellón and A. Jiménez-López, Appl. Catal., B, 2010, 97, 257–268 CrossRef.
- K. Chandra Mouli, K. Soni, A. Dalai and J. Adjaye, Appl. Catal., A, 2011, 404, 21–29 CrossRef CAS.
- V. Sundaramurthy, I. Eswaramoorthi, A. K. Dalai and J. Adjaye, Microporous Mesoporous Mater., 2008, 111, 560–568 CrossRef CAS.
- D. Valencia and T. Klimova, Catal. Today, 2011, 166, 91–101 CrossRef CAS.
- S. Badoga, R. V. Sharma, A. K. Dalai and J. Adjaye, Fuel, 2014, 128, 30–38 CrossRef CAS.
- E. J. M. Hensen, P. J. Kooyman, Y. van der Meer, A. M. van der Kraan, V. H. J. de Beer, J. A. R. van Veen and R. A. van Santen, J. Catal., 2001, 199, 224–235 CrossRef CAS.
- G. Roussy and J. A. Pearce, in Foundations and industiral applications of microwaves and radio frequency fields, John Wiley & Sons, 1995 Search PubMed.
- Y. Suttisawat, H. Sakai, M. Abe, P. Rangsunvigit and S. Horikoshi, Int. J. Hydrogen Energy, 2012, 37, 3242–3250 CrossRef CAS.
- Z. Chemat-Djenni, B. Hamada and F. Chemat, Molecules, 2007, 12, 1399–1409 CrossRef CAS PubMed.
- S. Horikoshi and N. Serpone, Catal. Sci. Technol., 2014, 4, 1197–1210 CAS.
- T. Huang, W. Huang, J. Huang and P. Ji, Fuel Process. Technol., 2011, 92, 1868–1875 CrossRef CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- C. Ochoa-Hernández, Y. Yang, P. Pizarro, V. A. de la Peña O'Shea, J. M. Coronado and D. P. Serrano, Catal. Today, 2013, 210, 81–88 CrossRef.
- Y. Du, S. Liu, Y. Zhang, F. Nawaz, Y. Ji and F.-S. Xiao, Microporous Mesoporous Mater., 2009, 121, 185–193 CrossRef CAS.
- K. Chandra Mouli, S. Mohanty, Y. Hu, A. Dalai and J. Adjaye, Catal. Today, 2013, 207, 133–144 CrossRef CAS.
- S. Badoga, A. K. Dalai, J. Adjaye and Y. Hu, Ind. Eng. Chem. Res., 2014, 53, 2137–2156 CrossRef CAS.
- S. Wu, Y. Han, Y.-C. Zou, J.-W. Song, L. Zhao, Y. Di, S.-Z. Liu and F.-S. Xiao, Chem. Mater., 2004, 16, 486–492 CrossRef CAS.
- P. Bhange, D. S. Bhange, S. Pradhan and V. Ramaswamy, Appl. Catal., A, 2011, 400, 176–184 CrossRef CAS.
- T. E. Klimova, D. Valencia, J. A. Mendoza-Nieto and P. Hernández-Hipólito, J. Catal., 2013, 304, 29–46 CrossRef CAS.
- D. Zhang, A. Duan, Z. Zhao, X. Wang, G. Jiang, J. Liu, C. Wang and M. Jin, Catal. Today, 2011, 175, 477–484 CrossRef CAS.
- E. W. Qian, N. Chen and S. Gong, J. Mol. Catal. A: Chem., 2014, 387, 76–85 CrossRef CAS.
- (a) E. Siores and D. Do Rego, J. Mater. Process. Technol., 1995, 48, 619–625 CrossRef; (b) Y. Suttisawat, H. Sakai, M. Abe, P. Rangsungvigit and S. Horikosji, Int. J. Hydrogen Energy, 2012, 37, 3242–3250 CrossRef CAS.
- A. R. von Hippel, Dielectric materials and applications, John Wiley & Sons, New York, 1954 Search PubMed.
- X. Zhang, D. O. Hayward, D. Michael and P. Mingos, Chem. Commun., 1999, 975–976, 10.1039/A901245A.
- A. Corma, M. S. Grande, V. Gonzalez-Alfaro and A. V. Orchilles, J. Catal., 1996, 159, 375–382 CrossRef CAS.
- J. P. Dacquin, A. F. Lee, C. Pirez and K. Wilson, Chem. Commun., 2012, 48, 212–214 RSC.
- O. Y. Gutiérrez, K. A. Romero, G. A. Fuentes and T. Klimova, in Studies in Surface Science and Catalysis, ed. E. M. Gaigneaux and P. Ruiz, Elsevier, 2006, vol. 162, pp. 355–362 Search PubMed.
- O. Y. Gutiérrez, D. Valencia, G. A. Fuentes and T. Klimova, J. Catal., 2007, 249, 140–153 CrossRef.
- S. Zeng, J. Blanchard, M. Breysse, Y. Shi, X. Shu, H. Nie and D. Li, Microporous Mesoporous Mater., 2005, 85, 297–304 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |