Novel isatin derivatives of podophyllotoxin: synthesis and cytotoxic evaluation against human leukaemia cancer cells as potent anti-MDR agents
Abstract
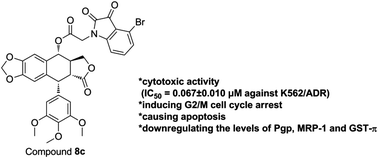
Multidrug resistance (MDR) is a major cause of chemotherapy failure in cancer therapy. In this study, a series of isatin derivatives of podophyllotoxin were synthesized and evaluated for their cytotoxic activity against human leukemia K562 cells and adriamycin-resistant K562/ADR cells using CCK-8 assay in vitro. All derivatives exhibited higher potency of antiproliferative effects against K562 and K562/ADR cell lines than the control drugs etoposide and adriamycin at nanomolar range, and markedly reduced the resistant factors. Among them, the cytotoxicities exhibited by compounds 8c and 8i were found to comparable or more effective than podophyllotoxin. In particular, 8c exhibited significant cytotoxicity against resistance K562/ADR cells with IC50 value of 0.067 ± 0.010 μM. Furthermore, cell cycle analysis revealed that 8c could remarkably induce K562/ADR cell cycle arrest in the G2/M phase. Meanwhile, the effect of 8c on apoptosis inducing was also observed notably by flow cytometry and Hoechst 33342 staining. Moreover, western blotting analysis suggested that 8c had the potential to overcome the resistance of K562/ADR cells by down-regulating the expression levels of multi-drug resistance-related proteins, such as Pgp, MRP-1 and GST-π.

 Please wait while we load your content...
Please wait while we load your content...