Fabrication of highly fluorescent CdSe quantum dots via solvent-free microfluidic spinning microreactors†
Abstract
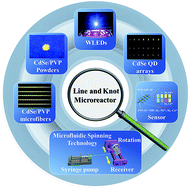
A method for in situ fabrication of highly fluorescent hybrid materials prepared via microfluidic spinning technology (MST) has been demonstrated. Initially, we applied MST to construct one dimensional microfibers, then utilized line–line junctions and knots as the microreactors, in situ synthesizing highly fluorescent CdSe quantum dot (QD) hybrids through solid–solid contact reaction. After grinding the as-prepared CdSe hybrid fibers to powders, we facilely used them as the phosphor to successfully prepare a white light-emitting diode (WLED). Also, we applied the CdSe QD arrays as “test paper” for detection of Pb2+ and Cu2+ content, which are similar to “touch spots” of a neural network. This strategy shows not only green synthesis of fluorescent QD hybrids, but also a great prospect for large scale synthesis of fluorescent materials with energy saving.

 Please wait while we load your content...
Please wait while we load your content...