Synthesis of polyacrylamides hydrophobically modified with butyl acrylate using a nanoclay with interlayer spaces for butyl acrylate aggregation: studies on the microstructure and aqueous solution viscosity
Mahdi Abdollahi* and
Hossein Khakpour
Polymer Reaction Engineering Department, Faculty of Chemical Engineering, Tarbiat Modares University, P.O.Box: 14115-114, Tehran, Iran. E-mail: abdollahim@modares.ac.ir; Tel: +98 21 82884959
First published on 26th November 2015
Abstract
Aqueous solution free-radical copolymerization of acrylamide with hydrophobic butyl acrylate (BA) was performed by potassium persulfate as an initiator in the presence of nanoclay. The effect of two nanoclays with different natures, i.e. hydrophilic Cloisite Na+ and hydrophobic Cloisite 30B, on the microstructure and aqueous solution viscosity of the synthesized copolymers were studied. It was found from microstructural studies by NMR that copolymerization with Cloisite Na+ may proceed via a mechanism similar to the heterogeneous mechanism, while those with Cloisite 30B may proceed simultaneously with both micellar and heterogeneous mechanisms with a relatively high tendency toward the micellar method. These findings were further confirmed by the water solubility, XRD, TEM, dynamic light scattering (DLS) and viscosity analyses. Significant intercalation of the chains into the clay galleries was observed only with Cloisite Na+. The results of the DLS analysis as well as the aqueous solution viscosity versus copolymer composition, NaCl concentration and temperature revealed intermolecular aggregation of the BA groups especially for multiblock structured copolymers synthesized with an emulsifier or Cloisite 30B.
Introduction
Due to its high molecular weight, polyacrylamide (PAM) is an interesting polymer as a thickening agent and rheological modifier in aqueous solutions.1–4 To improve rheological properties such as resistance to the shear rate, salt tolerance and thermal stability, PAMs have been hydrophobically modified by incorporating a small amount of hydrophobic comonomers into the main chain, resulting in water-soluble polymers so called hydrophobically modified polyacrylamides (HMPAMs).2–5 Linear alkyl or alkylaryl acrylamides and alkyl acrylates are commonly used hydrophobic comonomers.1,2,4 These polymers have found applications in various fields such as enhanced oil recovery, drilling fluid, coatings and paints.2,4 To be applicable in the above-mentioned applications, synthesized HMPAMs should have suitable rheological properties such as thickening behavior with increasing polymer concentration in the aqueous solution, shear-thinning behavior, resistance to the shear fields and stability against high concentration of the salts and high temperature to maintain their viscosities.1–8There is a direct relationship between the thickening and rheology modification properties and chemical microstructure of the HMPAMs.2–5 Molecular weight and its distribution and copolymer composition and its distribution (i.e. comonomers sequence length) are of important (micro)structural characteristics which have significant effects on the association of hydrophobic groups and rheological properties.1–5,8–10 Mark–Houwink relationship established for the PAM homopolymer can be used to estimate molecular weight of the HMPAMs where mole fraction of the hydrophobic comonomer in the copolymer is very low relative to the hydrophilic main monomer, i.e. acrylamide.2,8–12 On the other hand, 1H-NMR method can give reliable results on the copolymer composition providing that mole fraction of the hydrophobic comonomer incorporated into the copolymer chains is high enough (>2 mole%) or hydrophobic comonomer contains two terminal methyl groups (N,N-dihexylacrylamide, for example).2,5,13
There are different methods to synthesize HMPAMs such as heterogeneous, homogeneous and micellar copolymerizations.1–5,13 HMPAMs can be synthesized via free-radical copolymerization technique without any additive (heterogeneous method).5,13 On the other hand, it is possible to dissolve the hydrophobic comonomer in the aqueous solution using a co-solvent (homogeneous method) or an emulsifier (micellar method).2,5,13 Studies on the heterogeneous and homogeneous methods have revealed that hydrophobic comonomer incorporates randomly into the copolymer chain with a sequence length equal to about unity. On the other hand, emulsifier with a concentration much higher than its critical micelle concentration (CMC) is used in the micellar method; resulting in the hydrophobic monomer swollen micelles. The entrance into, propagate inside and exit from the micelles of the propagating macroradicals in the aqueous solution can repeat several times for a single propagating macroradical before its termination, resulting in a multi-block microstructure.2,4,13 It can be attributed to a relatively high lifetime of propagating polyacrylamide macroradicals due to the high ratio of the propagation to termination rate constants and to the small chain transfer constant in the water.1
Copolymerization of the acrylamide (AM) and a small amount of fluorinated (meth)acrylate has been reported for synthesizing HMPAMs where association behavior of the hydrophobic groups has been observed.14 Preparation of hydrophobically modified polyelectrolytes by precipitation copolymerization of acrylic acid and 3-[tris(trimethylsilyloxy)silyl] propyl methacrylate in the supercritical carbon dioxide has been reported.15 Acrylic acid/alkyl acrylate (alkyl chain with a length of 8, 12, 14, 16 or 18) copolymer has been synthesized via precipitation polymerization and a random distribution of the alkyl acrylate in the copolymer has been reported.16 These copolymers exhibited strong association behavior in the aqueous solution.
Although homogeneous and microemulsion radical copolymerization of AM and n-butyl acrylate (BA) has been reported;17–20 however, these studies have not intended to prepare water-soluble HMPAMs or study their physical properties in the aqueous solutions. More recently, two works has been reported on the use of wide accessible, low cost and hydrophobic BA comonomer for synthesis of the HMPAMs.13,21 The aggregation behavior in aqueous solution of hydrophobically modified polyacrylates synthesized by atom transfer radical polymerisation (ATRP) of a mixture of alkyl acrylate and t-butyl acrylate (tBA) and subsequent hydrolysis of the tBA has been investigated.21 The hydrophobicity of polyacrylates has systematically been varied by the amount of alkyl acrylate and in particular by the length of the hydrocarbon moiety where n-butyl, hexyl, isooctyl, and dodecyl acrylates have been used. Acrylates were randomly distributed along the polyelectrolyte backbone. Results showed formation of the hydrophobic aggregation domains whose size increases with the length of the alkyl chain.
AM and BA have been copolymerized via heterogeneous and micellar methods in the aqueous solution without and with, respectively, SDS as an emulsifier13 and BA-modified PAMs with random and multi-block, respectively, microstructures with molecular weights in the range of about 106 g mol−1 have been prepared. Results showed that it is possible to establish a reasonable microstructure-water solubility relationship for the AM/BA copolymers synthesized by different methods. For example, length of BA blocks per micelle higher than about 10 was found to limit water solubility of the copolymers.
Beneficial effect of the organically modified clays, i.e. Cloisite 30B, on the polymerization rate of controlled radical polymerization of (meth)acrylates has been reported.22–24 These observations have been found to be due to a definite interaction between the hydroxyl moiety (Al–O–H) of organoclay and carbonyl group (〉C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the (meth)acrylats.22–24 Therefore, (meth)acrylates can diffuse into the clay galleries, resulting in the increased basal spacing (d001) of the organoclay.
O) of the (meth)acrylats.22–24 Therefore, (meth)acrylates can diffuse into the clay galleries, resulting in the increased basal spacing (d001) of the organoclay.
According to the above explanations, interlayer spaces of organoclays are expected to be suitable loci for association of the hydrophobic interacting (meth)acrylates such as BA. On the other hand, due to the fully exfoliation of layers,25,26 pristine clays cannot provide spaces for BA association; hence, random distribution with a sequence length of about 1 for BA is expected. Hence, in continuum of the previous work,13 AM and BA are copolymerized in the present work via free radical aqueous solution copolymerization in the presence of nanoclay. The aim of this work is to investigate effect of the dispersion state of the two different nanoclays, i.e. hydrophilic Cloisite Na+ pristine clay with a fully exfoliation of the layers in the aqueous solution25,26 or hydrophobic Cloisite 30B organoclay with a slightly intercalation of the layers only by BA,22–24 on the copolymerization mechanism, microstructure and aqueous solution viscosity, i.e. establishing the synthesis–structure–property relationship, of the AM/BA copolymers. Accordingly, chemical structure of the synthesized copolymers is characterized by 1H NMR and 13C NMR spectroscopies. Then, microstructure and hydrophobic aggregation behavior are then evaluated using water solubility test, 1H NMR spectroscopy (for copolymer composition determination), intrinsic viscosity measurement (for calculation of the molecular weight) and dynamic light scattering (DLS) (for calculation of the hydrodynamic radius, Rh, of the copolymer chains in the aqueous solution). Morphology of the polymer/clay (nano)composites is investigated by X-ray diffraction (XRD) and transmission electron microscopy (TEM). Viscosity of the aqueous solutions containing AM/BA copolymers is investigated as a function of copolymer concentration, sodium chloride (NaCl) concentration and temperature.
Experimental
Materials
The AM (Merck, for synthesis) was recrystallized twice in the acetone. BA (Merck, for synthesis) was purified via passing through a basic alumina column. Potassium persulfate (KPS) from Merck were used without further purification. Cloisite Na+ and Cloisite 30B (both from Southern Clay Products Inc.) as a pristine and organically modified (modifier: (bishydroxyethyl)methyl tallow quaternary ammonium cation) nanoclays, respectively, with cation exchange capacities of 92 and 90 meq./100 g clay, respectively, were dried under reduced pressure at 70 °C for 24 h before use. NaCl (purity > 99%) and acetone (purity > 99%) were purchased from Dr Mojallali Co. (Iran). Deionized water and nitrogen (purity > 99.9%) were used in all experiments.Synthesis
In the previous research, micellar (experiment AB-1) and heterogeneous (experiment AB-2) methods were used for synthesis of the AM/BA copolymers where SDS (1.2 g in the case of micellar method) and KPS (0.05 wt% relative to the aqueous phase in the both methods) were employed as emulsifier and initiator, respectively.13 1.056 g (93 mol%) and 0.144 g (7 mol%) of AM and BA, respectively were used in the above-mentioned reactions. In the present work, similar procedure of the micellar copolymerization was also used except that nanoclay was used instead of the SDS (Table 1). A 50 ml ampoule containing 40 ml deionized water was used as a reaction chamber. By using a magnet stirrer (in the case of Cloisite Na+) or ultrasonic homogenizer (in the case of Cloisite 30B) for 30 min, the predefined amount of clay (Table 1) was dispersed in the aqueous phase and given amounts of the AM (1.056 g) and BA (0.144 g) were then added to the reaction mixture. The resulting mixture was further mixed for 1 h. It has been reported that layers of the Cloisite Na+ can readily be separated to single layers in the water with a concentration as high as 10 wt%, forming a stable dispersion for a long time period.25,26 However, Cloisite 30B is a hydrophobic clay and precipitates immediately in the aqueous phase; hence, it was dispersed in the aqueous phase in the presence of hydrophobic BA comonomer by using an ultrasonic homogenizer.| Exp. no. | Cloisite | SDS (g) | Conv. (%) | Water solubilityb | Method | |
|---|---|---|---|---|---|---|
| Na+ (g) | 30B (g) | |||||
| a All reactions were performed with 1.056 g (93 mol%) and 0.144 g (7 mol%) of AM and BA, respectively. KPS amount was kept constant at 0.05 wt% relative to the aqueous phase. Total monomer concentration was selected to be 3 wt% relative to the aqueous phase.b Excellent solubility (++) and good solubility (+). | ||||||
| ABC-1 | 0.6 | — | 0.6 | 78 | + | With SDS and Cloisite Na+ |
| ABC-2 | 1.2 | — | — | 71 | ++ | With Cloisite Na+ |
| ABC-3 | — | 1.2 | — | 74 | ++ | With Cloisite 30B |
The reaction mixture was immersed in a water/ice bath followed by bubbling with nitrogen for 30 min under magnetic stirring to remove air, and then a given amount of KPS was added (Table 1). The reaction chamber was immersed in a 50 °C water bath for 8 h while reaction mixture was mixed with a magnetic stirrer, after which, copolymer and clay were precipitated together by adding excess amount of the acetone. The precipitated product was again dissolved in the water, then precipitated using the excess acetone and dried in a vacuum oven at 60 °C for 24 h. Dried product was directly subjected to the XRD analysis. To separate copolymer and clay, dried product was dissolved in the water; clay was then precipitated by an ultracentrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and separated by filtration from upper clear aqueous solution containing copolymer. Finally, copolymer was precipitated from clear aqueous solution by adding excess amount of the acetone, filtered, and dried under vacuum at 60 °C for 24 h. Purified copolymer was then used in the NMR, DLS and viscosity measurements. Conversion of the AM and BA monomers to the copolymer was measured gravimetrically.
000 rpm and separated by filtration from upper clear aqueous solution containing copolymer. Finally, copolymer was precipitated from clear aqueous solution by adding excess amount of the acetone, filtered, and dried under vacuum at 60 °C for 24 h. Purified copolymer was then used in the NMR, DLS and viscosity measurements. Conversion of the AM and BA monomers to the copolymer was measured gravimetrically.
Characterization
To study the copolymer composition and chemical microstructure, 1H-NMR and 13C-NMR spectra were recorded using Bruker Avance 400 MHz NMR spectrophotometer. Purified copolymer with a concentration of about 3 wt% was dissolved in the D2O solvent and used for analysis.Water solubility of the purified copolymers was evaluated visually via dissolution of a given amount of copolymer in the water where rapid and slow dissolutions were defined as excellent and good, respectively, water solubility.
Ubbelohde viscometer was used for the intrinsic viscosity measurement at 25 °C, from which molecular weight of the copolymers was estimated. The intrinsic viscosity ([η]) and Huggins equation coefficient (KH) can be calculated from experimental viscosity data of polymeric solutions over various concentrations using eqn (1).13 Average efflux time for the solvent or the polymer solution was obtained from at least three measurements. Confidence intervals for the measured efflux times were in the range of ±1 s.
Due to the low concentration of BA in comparison with that of AM in the initial feed and thereby in the copolymer chain, the Mark–Houwink equation proposed for PAM (eqn (2)) in a 0.1 M solution of NaCl at 25 °C (ref. 1 and 27) was used for molecular weight estimation of the copolymers.
 | (1) |
| [η] = 9.33 × 10−3Mw0.75 | (2) |
Under same conditions, aqueous solution viscosity of the HMPAMs is affected by both the molecular weight and intermolecular association, originated from the hydrophobic BA segment's association, of the HMPAMs. To determine extent of the intermolecular association, DLS technique (Model Malvern Zetasizer Nano S90) was used to determine hydrodynamic radius (Rh) of the copolymer chains in the aqueous solution. Then, individual influence of the molecular weight and intermolecular association on the aqueous solution viscosity can be evaluated, which will be discussed later.
Dilute aqueous solutions (with concentrations lower than 0.1 wt%) were prepared and centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to remove any possible impurities. The tests were carried out at 25 °C by a laser light with a wave length of 633 nm, where viscosity of the solution and refractive indexes of the solvent and solution were used as input data in the calculations. DLS technique measures in fact the translational diffusion coefficient (D) of polymer chains, from which hydrodynamic radius of polymer coils can be calculated by considering the Stokes–Einstein equation (eqn (3)).28
000 rpm to remove any possible impurities. The tests were carried out at 25 °C by a laser light with a wave length of 633 nm, where viscosity of the solution and refractive indexes of the solvent and solution were used as input data in the calculations. DLS technique measures in fact the translational diffusion coefficient (D) of polymer chains, from which hydrodynamic radius of polymer coils can be calculated by considering the Stokes–Einstein equation (eqn (3)).28
 | (3) |
X-ray diffraction (XRD) patterns were collected on an X-ray diffraction instrument (Philips, Model X'Pert MPD) with a cobalt tube (λ = 0.179 nm) as a anode at the room temperature. The system consists of a rotating anode generator operated at 40 kV and 30 mA current. The samples were scanned from 2θ = 1° to 12° at the step scan mode, and the diffraction pattern was recorded using a scintillation counter detector. The distribution of clay layers into the polymer matrix was observed by a TEM (Philips, Model CM-30) operated at an accelerated voltage of 300 kV. Dried product containing a mixture of copolymer and clay was dispersed in the deionized water for 24 h and then one drop of the diluted suspensions was suspended on a carbon-coated copper grid, vacuum dried and subjected to the TEM observation.
Results and discussion
In the previous work,13 heterogeneous (without any additive, Fig. 1a) and micellar (with SDS, Fig. 1b) methods were used for synthesis of the random and multi-block, respectively, AM/BA copolymers. Random distribution of the BA with a microstructure form of the [(AM)n-co-(BA)m]p with a BA sequence length of about 1 (m = 1) was observed in the heterogeneous method while both random and multi-block distributions of the BA with a microstructure form of the [(AM)n-co-(BA)m]x-b-(BA)y]z (m = 1) was observed for the copolymers formed via micellar method (Fig. 2).13 | ||
| Fig. 2 Schematic representation of the free radical aqueous solution copolymerization of AM and BA in the presence of various additives and possible microstructures of the produced copolymers. | ||
Aim of the present work is to investigate effect of the layered nanoclay on the behavior of the aqueous phase copolymerization of the AM and BA. Depending on the hydrophobicity nature of the clay used, it seems that different loci in the aqueous medium can be considered for aggregation of the water-insoluble portion of the BA. When the hydrophilic Cloisite Na+ is used, it can rapidly separate to single layers in the water,25,26 therefore, there are no clay galleries for aggregation of the hydrophobic species. Thus, single layers can physically adsorb on the surface of hydrophobic BA droplets, producing a system so called clay-stabilized Pickering emulsion.29,30 It is expected that reaction in such system proceeds with a pattern similar to the heterogeneous method (Fig. 1c), producing a copolymer with a general formula of [(AM)u-co-(BA)v]w (Fig. 2). On the other hand, when organically modified clay (Cloisite 30B in the present work) is used in the aqueous medium, interlayer spaces of the clay can provide suitable loci for aggregation of the hydrophobic species (BA in the present case) (Fig. 1d).22–24 Therefore, copolymerization of AM and BA in such system is expected to proceed with a pattern similar to the micellar method. However, block length of the BA incorporated into the copolymer chains may be different from that of the micellar method due to the different number of sites available for the BA aggregation. Hence, microstructure of the [(AM)p-co-(BA)q]r-b-(BA)s]t can be considered for the produced copolymers (Fig. 2).
Microstructural characterization
Structure of the AM/BA copolymers synthesized under various condition (Table 1) were analyzed from corresponding 1H-NMR (Fig. 3) and 13C-NMR (Fig. 4) spectra. D2O was used as a solvent. All peaks appeared in the 1H-NMR and 13C-NMR spectra were assigned to the corresponding protons and carbons, respectively.13 Due to their favorable water solubility, copolymer ABC-3 synthesized with Cloisite 30B was used in the 13C-NMR for further characterization of the copolymer structure. | ||
| Fig. 3 1H-NMR spectra of AM/BA copolymers prepared under various conditions (Table 1) in the D2O solvent along with assigning peaks to the corresponding protons. | ||
 | ||
| Fig. 4 Proton-decoupled 13C-NMR spectra recorded for copolymer ABC-3 in the D2O solvent along with assigning peaks to the corresponding carbons. | ||
Reaction ABC-1 was carried out in the presence of an equal amount of the SDS and Cloisite Na+ where amount of SDS decreased to half the amount of that in the reaction AB-1. Due to the presence of SDS emulsifier, reaction ABC-1 is still expected to proceed via a micellar mechanism similar to the reaction AB-1 (for more details, see the next paragraph) except that BA block length per micelle (NH) can be different and molecular weight can be affected by the presence of nanoclay, which will be discussed later. This similarity can be observed from signal of the methyl protons of BA at about 0.8 ppm (Fig. 3) where it has been appeared as a relatively broad peak without spin–spin splitting in both reactions. It can be attributed to the high mole fraction of BA incorporated into the chain (case of copolymer AB-1) and/or high value of NH (case of ABC-1) (Table 2), resulting in the microscopic phase separation in the aqueous solution via the hydrophobic intermolecular aggregation (see next sections).
| Exp. no. | fAM | fBA | FAM | FBA | NH |
|---|---|---|---|---|---|
| a Theoretical NH for experiment ABC-1 was calculated according to the previous work13 by only considering the amount of SDS in the initial reaction mixture. It should be mentioned that interactions between the SDS and Cloisite Na+ can change ability of SDS molecules to form micelle.31–33 | |||||
| ABC-1 | 0.93 | 0.07 | 0.966 | 0.034 | 33.8a |
| ABC-2 | 0.93 | 0.07 | 0.984 | 0.016 | — |
| ABC-3 | 0.93 | 0.07 | 0.941 | 0.059 | — |
Mole fraction of the BA in the AM/BA copolymer (FBA) can be calculated from corresponding signal intensities of the BA and AM protons in the 1H-NMR spectra using the following equation:
 | (4) |
On the other hand, FBA value of copolymer ABC-1 is expected to be close to that of the copolymer AB-1; however, different results were observed (Table 2). FBA value for copolymer ABC-1 is much lower than that of copolymer AB-1, but still is higher than that of copolymer ABC-2. It may be attributed to the coverage of BA swollen micelles' surface with clay layers, as discussed above, and/or effect of the layered Cloisite Na+ on the ionic strength of the aqueous medium and thereby on the CMC value of SDS.31–33 Due to the repulsion between the anion charges of SDS and Cloisite Na+, full coverage of the surface of BA swollen micelles by clay layers may not be occurred; therefore, micellar copolymerization will occurred somewhat.31 CMC value of the SDS has been observed to decrease significantly in the presence of pristine sodium montmorillonite (i.e. Cloisite Na+).32,33 Without Cloisite Na+, the CMC of SDS in the water was 8.32 mM. In the presence of 1 wt% Cloisite Na+, this value decreased to 6.4 mM.33 The decrease of the CMC in the presence of Na-MMT increases the number of micelles for a given emulsifier concentration, resulting in the decreased number of hydrophobic monomers (BA in the present work) per micelles (NH) (see eqn (5) in the next section). Therefore, by entrance of propagating macroradical into the micelle, number of the BA consumed in the BA swollen micelle decreases, resulting in the decreased FBA value.
For copolymers AB-2, ABC-2 and ABC-3, signal of the BA methyl protons at about 0.8 ppm has been appeared as a triplet peak (Fig. 3), indicating clearly spin–spin splitting of protons in the –CH2–CH3 group. Among three reactions performed in the presence of nanoclay, maximum FBA value was obtained for copolymer ABC-3 to be 0.059 (Table 2), which is higher than that of copolymer AB-2 prepared by heterogeneous method and is lower than that of copolymer AB-1 prepared by micellar method. It means that reaction ABC-3 may proceed simultaneously via two different mechanisms of the heterogeneous and micellar methods. The proposed copolymerization mechanism will further be verified by aqueous solution viscosity and DLS studies on the intermolecular association of BA segments, which will be discussed later. As already mentioned, interlayer spaces of organoclay are desirable loci for aggregation of the hydrophobic species (BA in the present case). By consuming BA in the aqueous medium, BA may exit from organoclay galleries and diffuse to the bulk of aqueous medium. Thus, reaction proceeds via heterogeneous mechanism. However, there is also semi-micellar mechanism for BA to be consumed. Propagating macroradicals can also enter into the interlayer spaces of the BA swollen organoclays and grow by consuming the BA comonomers. Therefore, two different roles in the reaction can be considered for Cloisite 30B organoclay. Consequently, compared to the micellar method with emulsifier, a multi-block structure with a shorter BA block length (s < y) and a higher ratio of random to block distribution of BA (r/s > x/y) is expected (Fig. 2). Random copolymer can also be considered as a limiting case of the multi-block structure when s or y value approaches to the unit value. This situation may occur for reactions only performed with a very high concentration of SDS or Cloisite 30B, which is not the case in the present work. For example, a random copolymer may be expected for micellar method when the high ratio of emulsifier to hydrophobic comonomer is used in such a way that each micelle contains only a single hydrophobic comonomer (i.e., NH = 1, see the next section).
Table 3 shows the intrinsic viscosity [η], Huggins equation coefficient (KH) and molecular weight of the copolymers ABC-1, ABC-2 and ABC-3; the corresponding values for (co)polymers PAM, AB-1 and AB-2 have been reported to be 199.0, 0.87 and 5.89 × 105, 106.5, 1.32 and 2.56 × 105 and 404.3 ml g−1, 0.31 and 1.52 × 106 g mol−1, respectively.13 It is clear from these data that among the other copolymers, copolymer AB-2 possesses highest molecular weight while a minimum molecular weight of 2.56 × 105 g mol−1 was observed for copolymer AB-1. Molecular weight of the copolymer decreased for reactions performed with the SDS, Cloisite Na+ or Cloisite 30B. It can be attributed to the chain transfer reactions occurred in the presence of clay32,34 and SDS.1,2 Impurities such as alcohol in the SDS and functional hydroxyl groups on the surface of clay can act as a chain transfer agent. Moreover, decrease in molecular weight of the copolymer prepared with Cloisite Na+ (copolymer ABC-2) is higher than that prepared with Cloisite 30B (copolymer ABC-3). It may be attributed to the higher surface area of the exfoliated structure of the Cloisite Na+ relative to the intercalated structure of the Cloisite 30B.
| Sample | [η] (ml g−1) | KH | Mw × 106 (g mol−1) |
|---|---|---|---|
| ABC-1 | 161.1 | 1.14 | 0.444 |
| ABC-2 | 281.8 | 0.84 | 0.937 |
| ABC-3 | 343.0 | 0.54 | 1.22 |
It is clear from Table 3 that intrinsic viscosity, KH value and molecular weights of copolymers ABC-1 and ABC-3 prepared with the SDS/Cloisite Na+ mixture and Cloisite 30B, respectively, are close to those of copolymers AB-1 and AB-2, respectively, prepared with and without, respectively, SDS.13 Again, these results verify structural similarities of the copolymer ABC-1 with copolymer AB-1 as well as the copolymer ABC-3 with copolymer AB-2 originated from similarities in the polymerization mechanism as discussed above (Fig. 1 and 2). It should be mentioned that copolymer ABC-2 with a minimum FBA value showed a KH value similar to that of the homopolymer PAM (Table 3). Difference in the viscosities can be attributed to the different molecular weights.
Relationship between the microstructure and water solubility of copolymers
The BA block length can be calculated by eqn (5) in terms of the number of hydrophobic monomers per micelles (NH).13–16
 | (5) |
Good and excellent water solubility has been observed for copolymers AB-1 and AB-2, respectively.13 Water solubility of the polymer decreases as the NH value increases. There would be a microstructure-related interpretation for the water solubility of polymers. In other words, water solubility of the multi-block copolymers decreases as the hydrophobic block length increases. Compared to sample AB-1, although theoretical NH value has increased two fold in the sample ABC-1, however, FBA value has decreased significantly. It means that copolymer ABC-1 may have a longer but fewer BA blocks than that of copolymer AB-1. Therefore, almost a similar behavior for water solubility of these samples was observed (Table 1). Copolymers AB-1 and ABC-1 were dissolved in the water completely but relatively slowly. It can be attributed to the intermolecular association network of relatively long BA block segments in the aqueous solution.
Copolymers AB-2 and ABC-2 were dissolved quickly in the water probably due to the random distribution of BA in the heterogeneous method. The almost similar result was also observed for sample ABC-3. It should be noted that after reaction, a stable dispersion of Cloisite Na+ in the aqueous medium was observed for reactions ABC-1 and ABC-2. Via ultracentrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, copolymer ABC-1 was easily separated from Cloisite Na+ while separation of the ABC-2 from Cloisite Na+ was very difficult, indicating that produced copolymers have different microstructures. It is clear from Table 2 that FBA in copolymer ABC-2 is significantly lower than that in copolymer ABC-1. The higher the mole fraction of BA incorporated into the AM/BA copolymer is, the easier the separation of hydrophobically modified PAM from hydrophilic Cloisite Na+ via ultracentrifugation will be. BA in the copolymer structure can decrease interactions between the both hydrophilic PAM chains and Cloisite Na+ layers. As expected in the case of reaction ABC-3, Cloisite 30B organoclay precipitates slowly without centrifugation in the stationary aqueous medium.
000 rpm, copolymer ABC-1 was easily separated from Cloisite Na+ while separation of the ABC-2 from Cloisite Na+ was very difficult, indicating that produced copolymers have different microstructures. It is clear from Table 2 that FBA in copolymer ABC-2 is significantly lower than that in copolymer ABC-1. The higher the mole fraction of BA incorporated into the AM/BA copolymer is, the easier the separation of hydrophobically modified PAM from hydrophilic Cloisite Na+ via ultracentrifugation will be. BA in the copolymer structure can decrease interactions between the both hydrophilic PAM chains and Cloisite Na+ layers. As expected in the case of reaction ABC-3, Cloisite 30B organoclay precipitates slowly without centrifugation in the stationary aqueous medium.
Analysis of the hydrophobic intermolecular aggregations
Hydrodynamic radius of the copolymer chains coil (Rh) in dilute solutions can be studied by DLS.13,28 Then, one would also be able to analyze the hydrophobic aggregations of the HMPAMs. Number and intensity size distribution curves are illustrated in Fig. 5. Bimodal distribution of size was observed in the intensity distribution curves (Fig. 5b). First distribution in the smaller sizes can be attributed to the copolymer chain coil with a slight and negligible intermolecular aggregation. Average value of the first distribution in the intensity distribution curves was in a good agreement with that in the number distribution curves (Fig. 5a and b). Therefore, it was considered to be close to the hydrodynamic radius of the copolymer chains coil (Rh). Number-average Rh values of copolymers ABC-1, ABC-2 and ABC-3 were obtained to be 6.6, 2.7 and 5.9 nm, respectively (Fig. 5a), while those of copolymers AB-1 and AB-2 have been reported to be 13.2 and 5.7 nm, respectively.13 Second distribution in the larger sizes (Fig. 5b) was attributed to the intermolecular aggregation of the hydrophobic groups from several copolymer chains, forming a large physical network. Intensity-average radius of hydrophobic aggregates of copolymers ABC-1, ABC-2 and ABC-3 were obtained to be 79.5, 46.6 and 73.2 nm, respectively (Fig. 5b), while those of copolymers AB-1 and AB-2 have been reported to be 220 and 100 nm, respectively.13 In the case of copolymer AB-2, this second peak was very weak and observed difficulty, indicating that there is no significant BA association.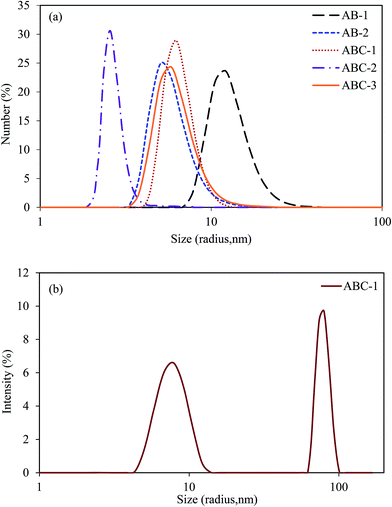 | ||
| Fig. 5 Number distribution (a) and intensity distribution (b) of copolymer coils and BA intermolecular aggregates versus size obtained from DLS analysis of the dilute aqueous solution. | ||
On the other hand, monomodal distribution of size was observed for all copolymers in the number distribution curves (Fig. 5a), indicating that number of the hydrophobic BA intermolecular aggregations in the dilute solution can be considered to be very small and almost negligible. BA molecules aggregate significantly via multi-block portions;13,21 hence, the size of BA aggregates in the copolymers AB-1, ABC-1 and ABC-3 was observed to be larger than that in the copolymers AB-2 and ABC-2. In other words, multi-block structure of the copolymer ABC-1 can be concluded from DLS results.
On the other hand, minimum hydrophobicity and hydrophobic intermolecular aggregation was observed for a copolymer with a minimum FBA value and random copolymer structure, i.e. copolymer ABC-2. Therefore, minimum Rh value is expected for sample ABC-2 (Fig. 5). The higher the hydrophobicity and hydrophobic intermolecular aggregation of the copolymers is, the higher the Rh value will be. Fig. 5 shows almost the same Rh value for copolymers AB-2, ABC-1 and ABC-3; which is lower than that of copolymer AB-1 (micellar method). NH value of copolymer ABC-1 is theoretically expected to be almost two times higher than that of copolymer AB-1 (Table 2); however, FBA value of copolymer ABC-1 is much lower than that of copolymer AB-1. As already mentioned, CMC value of the SDS decreases significantly in the presence of Cloisite Na+,32,33 resulting in the decreased value of the NH (see eqn (5)). Therefore, experimental NH value is lower than theoretical one in the copolymer ABC-1. Moreover, clay layers located on the surface of micelles can prevent entrance of the growing macroradicals into the micelles.31 Then, decrease in the FBA value of reaction ABC-1 is reasonable.1,2 By comparing Rh values and intermolecular aggregations obtained for copolymers AB-1 (micellar method), AB-2 (heterogeneous method) and ABC-3 (with Cloisite 30B), one may conclude that copolymerization of AM and BA can proceed simultaneously via both micellar and heterogeneous methods; however, micellar copolymerization is relatively predominant. It should be mentioned that when Rh values are compared, molecular weight of the copolymers should also be considered. DLS results are consistent with those observed from water solubility test and viscosity measurement.
Morphology of the polymer/clay nanocomposites
XRD analyses were used to investigate distribution states of the Cloisite Na+ or Cloisite 30B in the copolymer matrix. Results obtained from XRD patterns are given in Table 4, indicating basal spacing (d001) of 13.0 and 18.7 Å for pure Cloisite Na+ and Cloisite 30B, respectively. Basal spacing of Cloisite 30B is greater than that of Cloisite 30B due to the intercalation of galleries by organic modifier. Value of d001 in the copolymerization with Cloisite Na+ has increased from 13.0 Å for pure Cloisite Na+ to 22.1 and 21.2 Å for nanocomposites ABC-1 and ABC-2, respectively. It means that hydrophilic copolymers produced have been intercalated and located between the interlayer spaces of hydrophilic Cloisite Na+ under drying of the reaction mixture. It is reasonable because strong interaction between the polymer chains and clay is expected. On the other hand, as expected, no significant increase in the basal spacing of nanocomposite ABC-3 was observed. Slightly increase in the basal spacing may be attributed to entering macroradicals into the some Cloisite 30B galleries; however, its contribution is not significant. Hence, among two different mechanisms of copolymerization with Cloisite 30B discussed in the previous section, micellar mechanism seems to be relatively dominant.| Sample | Nanoclay used | Peak angle [2θ(°)] | Basal spacing (d001) (Å) |
|---|---|---|---|
| Cloisite Na+ | — | 7.897 | 13.0 |
| Cloisite 30B | — | 5.499 | 18.7 |
| ABC-1 | Cloisite Na+ | 4.643 | 22.1 |
| ABC-2 | Cloisite Na+ | 4.830 | 21.2 |
| ABC-3 | Cloisite 30B | 4.999 | 20.5 |
To further investigate its structure, nanocomposite ABC-2 was subjected to the TEM analysis and the corresponding micrographs are shown in Fig. 6. The clay layers appear as dark strips while the polymer matrix appears as a gray/white domain.26 It is clear from this image and XRD results that silicate layers are dispersed with a thickness of about 2–25 nm in the polymer matrix, indicating that both exfoliated and intercalated morphology may be formed, however, intercalated structure is dominant.
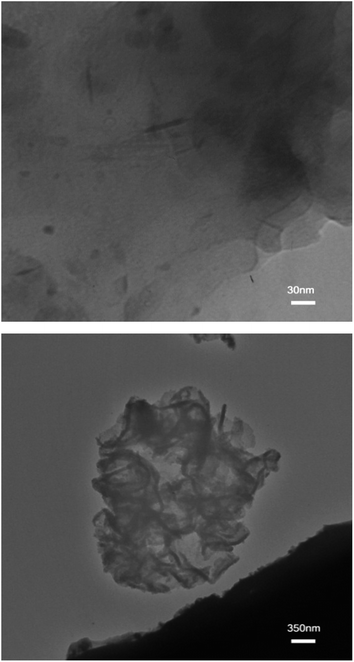 | ||
| Fig. 6 TEM micrographs with different magnifications of the copolymer/Cloisite Na+ nanocomposite prepared by reaction ABC-2. | ||
Aqueous solution viscosity of the AM/BA copolymers
 | ||
| Fig. 7 Aqueous solution reduced viscosity (ηred) as a function of AM/BA copolymer concentration at constant temperature of 25 °C. | ||
For copolymers synthesized with clay, maximum ηred value was observed for copolymer ABC-3. It may be attributed to the higher molecular weight of copolymer ABC-3 in comparison with the copolymers ABC-1 and ABC-2 as well as to the multiblock microstructure of the copolymer ABC-3 in comparison with the copolymer ABC-2. Therefore, one can conclude that both molecular weight of the copolymer and interchain aggregation of the hydrophobic groups can affect the aqueous solution viscosity of the hydrophobically associating copolymers.
Conclusion
Random and multi-block copolymers of AM and BA were successfully synthesized via free radical aqueous solution copolymerization in the presence of Cloisite Na+ and Cloisite 30B, respectively. Microstructural characteristics such as the molecular weight, copolymer composition and hydrophobic intermolecular aggregations were investigated by various techniques and results were compared with those obtained for copolymers prepared by heterogeneous and micellar methods in the previous work.13 Molecular weight of the copolymers decreased in the presence of nanoclays, showing that chain transfer reactions occur between the propagating macroradicals and species such as hydroxyl groups on the surface of nanoclays. Maximum and minimum values of FBA (and thereby hydrophobicity values) were deduced from corresponding 1H NMR spectra for the copolymers AB-1 and ABC-2, respectively, with multi-block and random, respectively, microstructures. Water solubility of the synthesized copolymers showed significant differences between the multi-block and random microstructures. Results obtained by DLS measurements indicated that intermolecular aggregations of the hydrophobic groups can also be found even in the dilute polymeric solutions. It was found from reduced viscosity measurements of the copolymers in the aqueous solution that BA-modified polyacrylamides especially those with a multiblock structure (i.e. copolymers AB-1 and ABC-3) are salt resistant and thermally stable, allowing us to use these copolymers as an efficient thickening agent of the aqueous solutions with a high salinity at elevated temperature. This behavior was attributed to the intermolecular aggregation of the hydrophobic BA units. Results showed that both the molecular weight and intermolecular aggregations of the HMPAMs should be considered to evaluate their rheological properties. XRD and TEM revealed intercalated structure as a dominant structure of the nanocomposites. Intercalation of clay was significant for a reaction performed with Cloisite Na+ while intercalation of the Cloisite 30B was not remarkable. It was concluded that aqueous solution copolymerization of the AM and BA in the presence of Cloisite Na+ proceeds similar to the heterogeneous mechanism with additional barrier effects of the clay on the BA diffusion from droplets to the aqueous phase and entrance of the propagating macroradicals into the BA droplets, while that in the presence of Cloisite 30B proceeds simultaneously with both the heterogeneous and micellar mechanisms but with a relatively high tendency toward micellar method. This work revealed that microstructure of the HMPAMs can be controlled using nanoclay, instead of SDS, as an additive. Before addition of the initiator to the reaction mixture, Cloisite 30B was allowed to intercalate with BA for 1 h. It seems that long time dispersion of the Cloiste 30B with the BA or any other hydrophobic (meth)acrylate in the aqueous solution prior to the polymerization22–24 may increase contribution of the micellar mechanism in the copolymerization of AM and (meth)acrylates.References
- A. Hill, F. Candau and J. Selb, Macromolecules, 1993, 26, 4521–4532 CrossRef CAS.
- F. Candau and J. Selb, Adv. Colloid Interface Sci., 1999, 79, 149–172 CrossRef CAS.
- E. Volpert, J. Selb and F. Candau, Polymer, 1998, 39, 1025–1033 CrossRef CAS.
- D. A. Z. Wever, F. Picchioni and A. A. Broekhuis, Prog. Polym. Sci., 2011, 36, 1558–1628 CrossRef CAS.
- E. Volpert, J. Selb and F. Candau, Macromolecules, 1996, 29, 1452–1463 CrossRef CAS.
- C. L. McCormick, J. C. Middleton and C. E. Grady, Polymer, 1992, 33, 4184–4190 CrossRef CAS.
- E. E. Kathmann, L. A. White and C. L. McCormick, Macromolecules, 1997, 30, 5297–5304 CrossRef CAS.
- T. Noda, A. Hashidzume and Y. Morishima, Macromolecules, 2001, 34, 1308–1317 CrossRef CAS.
- A. Sabhapondit, A. Borthakur and I. Haque, J. Appl. Polym. Sci., 2003, 87, 1869–1878 CrossRef CAS.
- Y. Feng, L. Billon, B. Grassl, A. Khoukh and J. François, Polymer, 2002, 43, 2055–2064 CrossRef CAS.
- C. L. McCormick, T. Nonaka and C. B. Johnson, Polymer, 1988, 29, 731–739 CrossRef CAS.
- N. Lai, W. Dong, Z. Ye, J. Dong, X. Qin, W. Chen and K. Chen, J. Appl. Polym. Sci., 2013, 129, 1888–1896 CrossRef CAS.
- H. Khakpour, M. Abdollahi and A. Nasiri, J. Polym. Res., 2015, 22, 189 CrossRef.
- Y. X. Zhang, A. H. Da, G. B. Butler and T. E. Hogen-Esch, J. Polym. Sci., Part A: Polym. Chem., 1992, 30, 1383–1391 CrossRef CAS.
- H. Zhang, K. Xu, H. Ai, D. Chen, L. Xv and M. Chen, J. Solution Chem., 2008, 37, 1137–1148 CrossRef CAS.
- D. Q. Zhuang, J. C. A. Da, Y. X. Zhang, R. Dieing, L. Ma and L. Haeussling, Polym. Adv. Technol., 2001, 12, 616–625 CrossRef CAS.
- J. Barton and I. Capek, Macromolecules, 2000, 33, 5353–5357 CrossRef CAS.
- A. S. Brar, M. Mukherjee and S. K. Chatterjee, Polym. J., 1998, 30, 664–670 CrossRef CAS.
- U. Yildiz, I. Capek, D. Berek, Y. Sarov and I. W. Rangelow, Polym. Int., 2007, 56, 364–370 CrossRef CAS.
- N. Yin and K. Chen, Polymer, 2004, 45, 3587–3594 CrossRef CAS.
- S. Riemer, S. Prevost, M. Dzionara, M. S. Appavou, R. Schweins and M. Gradzielski, Polymer, 2015, 70, 194–206 CrossRef CAS.
- H. Datta, N. K. Singha and A. K. Bhowmick, Macromolecules, 2008, 41, 50–57 CrossRef CAS.
- M. Abdollahi and M. A. Semsarzadeh, Eur. Polym. J., 2009, 45, 985–995 CrossRef CAS.
- M. Abdollahi and M. A. Semsarzadeh, Polym. Sci., Ser. B, 2012, 54, 247–258 CrossRef CAS.
- Y. Wang, H. Zhang, Y. Wu, J. Yang and L. Zhang, Eur. Polym. J., 2005, 41, 2776–2783 CrossRef CAS.
- A. Varamesh, M. Abdollahi and H. H. Khanli, Polym. Sci., Ser. A, 2013, 55, 115–120 CrossRef CAS.
- J. François, D. Sarazin, T. Schwartz and G. Weill, Polymer, 1979, 20, 969–975 CrossRef.
- S. Will and A. Leipertz, Int. J. Thermophys., 1995, 16, 433–443 CrossRef CAS.
- G. Lagaly, M. Reese and S. Abend, Appl. Clay Sci., 1999, 14, 83–103 CrossRef CAS.
- S. Fujii, Y. Eguchi and Y. Nakamura, RSC Adv., 2014, 4, 32534–32537 RSC.
- A. Hild, J. M. Sequaris, H. D. Narres and M. J. Schwuger, Prog. Colloid Polym. Sci., 1998, 111, 174–178 CAS.
- A. Bonnefond, M. Paulis and J. R. Leiza, Appl. Clay Sci., 2011, 51, 110–116 CrossRef CAS.
- C. S. Chern, J. J. Lin, Y. L. Lin and S. Z. Lai, Eur. Polym. J., 2006, 42, 1033–1042 CrossRef CAS.
- M. Farrokhi and M. Abdollahi, J. Polym. Res., 2014, 21, 593 CrossRef.
- Y. Guo, Y. Liang, X. Yang, R. Feng, R. Song, J. Zhou and F. Gao, J. Appl. Polym. Sci., 2014, 131, 40633 Search PubMed.
- G. Broze, Handbook of Detergents, CRC Press, London, 2011 Search PubMed.
- W. M. Kulicke, R. Kniewske and J. Klein, Prog. Polym. Sci., 1982, 8, 373–468 CrossRef CAS.
- C. Zhong, P. Luo, Z. Ye and H. Chen, Polym. Bull., 2009, 62, 79–89 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |



