Magnetic Fe3O4@MOFs decorated graphene nanocomposites as novel electrochemical sensor for ultrasensitive detection of dopamine
Abstract
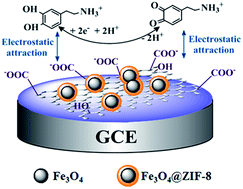
A novel hybrid nanocomposite of magnetic Fe3O4@ZIF-8 (zeolitic imidazolate framework-8 coated Fe3O4 nanocomposites denoted as Fe3O4@ZIF-8) decorated RGO (reduced graphite oxide) was prepared by a simple method for the first time and denoted as Fe3O4@ZIF-8/RGO. After the Fe3O4/RGO was formed by solvothermal approach, the MOFs (ZIF-8) was coated on the surface of Fe3O4 to get the Fe3O4@ZIF-8/RGO nanocomposite. The resulted Fe3O4@ZIF-8/RGO nanocomposite was characterized by means of the transmission electron microscope (TEM), scanning electron microscopy (SEM), Fourier transform infrared spectra (FT-IR), X-ray diffraction spectrometry (XRD), X-ray photoelectron (XPS), and vibrating sample magnetometer (VSM). This nanocomposite was modified on the glassy carbon electrode to fabricate biosensor which used to electrochemical determination for dopamine (DA) in phosphate buffer solution. The results demonstrated the fabricated biosensor showed great potential applications in the detection of DA with remarkable enhanced effect on voltammetric response of DA. The linear relationship between the response peak currents and DA concentration was in range from 2.0 × 10−9 to 1.0 × 10−5 M, the limits of detection is 6.67 × 10−10 M. Moreover, the prepared biosensor also showed good selectivity for DA detection in the presence of ascorbic acid and uric acid and satisfactory result in real samples detection.

 Please wait while we load your content...
Please wait while we load your content...