Pure phase orthorhombic MgTi2O5 photocatalyst for H2 production†
Ning Zhang,
Kaifu Zhang,
Wei Zhou,
Baojiang Jiang,
Kai Pan,
Yang Qu* and
Guofeng Wang*
Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education of The People’s Republic of China, Heilongjiang University, Harbin 150080, P. R. China. E-mail: copy0124@126.com; wanggf75@gmail.com
First published on 1st December 2015
Abstract
An undesirable phase transformation was overcome and pure phase orthorhombic MgTi2O5 nanocrystals were facilely prepared. The concentration of metal ions and the reaction temperature are the two factors that were found to affect the phase transformation of the magnesium titanates. The as-prepared pure phase MgTi2O5 nanocrystals possessed a high crystallinity and were 20 nm in diameter. They exhibited excellent photocatalytic hydrogen production activity (418 μmol h−1), and high apparent quantum efficiency (62.5% at 313 nm and 50.6% at 365 nm, respectively). Significantly, a “solar-to-hydrogen” efficiency of 0.52% was obtained. This may be attributed to the pure phase crystals and large surface area, which favored charge transfer and promoted photo-induced charge separations which is proven using photoelectrochemical measurements.
Introduction
Owing to the fantastic effects of semiconductors and powerful solar energy techniques, the dream of splitting water to produce hydrogen has come true.1 However, limited by photocatalyst materials, complex thermodynamics of the reactions and ambiguous mechanisms, the efficiency of photocatalytic H2 production is still very low, far from the requirement for industrialization. How to solve these thorny issues has become a challenge.2,3Alkali titanates with the formula M2TinO2n+1 have a unique layered and ilmenite or tunnel-like crystal structure, and have attracted increasing attention due to their more negative conduction band and high photocatalytic activity.4,5 Hereinto, MgTi2O5 possesses a suitable electronic structure and conduction/valence band position, that matches well with the redox potential of splitting water into hydrogen and oxygen molecules. MgTi2O5 has a theoretical band gap energy of approximately 3.4 eV, and contains Ti4+, a d0 metal ion which has the potential activity for water splitting.6 However, pure orthorhombic phase MgTi2O5 is very hard to prepare because the two main magnesium titanates, MgTiO3 and MgTi2O5, are associated minerals.7–10 These two phase transformations may be caused by possible crystallography incompatibilities, which may cause undesired secondary phases as often happened in the conventional solid state method to prepare magnesium titanates.11 However, pure phase materials are beneficial to photocatalysis because they reduce impurities and lead to fast charge transport from bulk to surface. Moreover, the synthesis of pure phase titanates is significant to, not only the properties themselves, but also to the inside study of their electronic energy structure. M. Morimoto reported the synthesis of uniformly porous MgTi2O5 with a narrow pore-size distribution at 1000–1200 °C and it showed excellent thermal expansion behavior.13 Recently, Deng and Chen et al. prepared a novel hybrid MgTi2O5–C nanocomposite from an in situ carbonization process of a cheap single molecule precursor with unique morphology that exhibited improved lithium ion battery properties.12 So, developing methods to prepare pure phase MgTi2O5 is significant to enhance its properties.
Generally speaking, if the phase transformation could be controlled or the MO6 units were “fixed”, pure phase magnesium titanates could be prepared. Luckily, some works have demonstrated that the concentration of metal ions can affect the phases of the products.14,15 Moreover, it was found in our previous works that parts of bivalent metals are easy to coordinate with some organic ligands (ethylene glycol) to form metal glycolates. Metal oxides or ilmenites were prepared using further calcinations. This means that pure phase MgTi2O5 could be prepared by fixing Mg2+ and Ti4+ using ethylene glycol and then calcination.
Hence, we report the facile synthesis of pure phase MgTi2O5 nanocrystals for H2 production. Different to the traditional method, we develop an organic ligand coordinated polymerization method. Ethylene glycol (EG) acts as a ligand to coordinate with Mg2+ and Ti4+ to form MgO6 and TiO6 units that are linked by EG, this can fix the cation distortion to avoid phase transformation and forms pure phase MgTi2O5 when calcined in air.
Experimental
Materials
Analytical grade Mg(CH3COO)2·4H2O, Ti(OC4H9)4, ethanol, methanol and ethylene glycol were used. All of the reagents and solvents were used as received without further purification. Distilled water was used throughout. For the preparation of the pure phase MgTi2O5, 0.0670 g of Mg(CH3COO)2·4H2O and 0.1062 mL of Ti(OC4H9)4 were dissolved in 30 mL of ethylene glycol, then the solution was stirred at room temperature for about 30 min. Subsequently, the obtained products were collected by means of centrifugation, washed with ethanol three times, and dried in air at 80 °C for 4 h; finally, they were sintered at 600 °C for 2 h in air, with a constant heating rate of 2 °C min−1. The yield of the products was about 45%. For easy distinguishability, pure phase MgTi2O5, mixed phase MgTiO3/MgTi2O5 and commercial MgTi2O5 were denoted as PMT, MMT and CMT, respectively.Characterization
The crystal structure was analyzed using X-ray powder diffraction (XRD) patterns that were obtained using a Bruker D8 Advance diffractometer using Cu Kα radiation (λ = 1.5406 Å, 40 kV, 40 mA). The size and morphology of the final products were investigated using scanning electron microscopy (SEM, Hitachi, S-4800) and transmission electron microscopy (TEM, JEOL, JEM-2010). Nitrogen adsorption–desorption isotherms were collected using a Tristar II 3020 surface area and porosity analyzer (Micromeritics). The pore size distribution plots were obtained using the Barrett–Joyner–Halenda (BJH) model. UV-vis absorption spectra were determined using a UV-vis spectrophotometer (Shimadzu UV-2550, Tokyo, Japan). Raman measurements were performed with a Jobin Yvon HR 800 micro Raman spectrometer at 457.9 nm. The laser beam was focused with a 50× objective lens onto a ca. 1 μm spot on the surface of the sample. X-ray Photoelectron Spectroscopy (XPS) tests were performed using Kratos-AXIS ULTRA DLD apparatus with an Al (mono) X-ray source, and the binding energies were calibrated with respect to the signal for adventitious carbon (binding energy = 284.6 eV).Photocatalytic H2 production experiment
The photocatalytic H2 evolution from water was conducted in an online photocatalytic hydrogen production system (AuLight, Beijing, China, CEL-SPH2N). A powder sample of the catalyst (0.1 g) was suspended in a mixture of 80 mL of distilled water and 20 mL of methanol in the cell using a magnetic stirrer. Pt-loaded photocatalysts were prepared by the known standard method of in situ photo-deposition. For this, the photocatalyst powder was added to an aqueous methanol solution containing the required amount (1 wt% of Pt) of H2PtCl6. The solution was illuminated for 3 h under a Xe lamp with an AM 1.5 filter (AuLight), filtered, and then dried in a static oven at 80–100 °C. Before the reaction, the mixture was deaerated using evacuation to remove the O2 and CO2 dissolved in the water. The reaction was carried out by irradiating the mixture with UV light from a 300 W Xe lamp with a 300–390 nm reflection filter which means the wavelength of light was approximately 300–390 nm. Gas evolution was observed only under photo-irradiation, this was analyzed using an online gas chromatograph (SP7800, thermal conductivity detector, molecular sieve 5 Å, N2 carrier, Beijing Keruida Limited, Beijing, China).Photoelectrochemical measurement
Photocurrent measurements were performed using a three-electrode configuration, with PMT, MMT and CMT films as the working electrode, saturated Ag/AgCl as the reference electrode, and platinum foil (3 × 2 cm) as the counter electrode. The working electrode films were prepared using the doctor-blade method, using a thin glass rod to roll a paste onto FTO to form a film (2 × 1 cm). The paste was prepared by stirring 0.2 g of photocatalyst powders in 0.5 mL of ethanol for at least 24 h. The films were annealed at 400 °C (ramp of 1 °C min−1) for 1 h to make them firm enough. Electrochemical impedance spectroscopy (EIS) measurements were performed in the dark and under visible light illumination (λ > 400 nm) in 1 M NaOH solution at open circuit voltage over a frequency range from 105 to 0.05 Hz with an AC voltage at 5 mV. The Mott–Schottky plots were obtained at a fixed frequency of 1 kHz in the dark.In addition, all of the data in this work, especially the photocatalytic activities and electrochemical measurements, were tested at least three times to confirm the reproducibility, to ensure the scientific and scrupulous treatment of our work.
Results and discussion
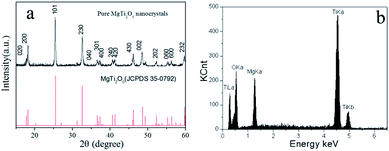
Different controlling experiments were done to prepare pure phase MgTi2O5 (PMT). As shown in Table S1,† when the polymerization temperature is low, only TiO2 is the final product. This shows that the coordination ability of Mg2+ and Ti4+ could be influenced by the reaction temperature. We can also learn from this, that the concentration affects the final products. Pure phase MgTi2O5 could only be synthesized at a low concentration and suitable polymerization temperature (17 °C). The higher the EG ratio (lower concentration), the more EG molecule polymerized in the metal glycolate that results in the strong fixation of the MO6 and TiO6 units.16The presence of the pure phase could be proven using the XRD patterns in Fig. 1a, as all of the diffraction peaks can be indexed to pure orthorhombic phase MgTi2O5 (JCPDS 35-0792). No other impurity peaks such as TiO2, MgTiO3 and Mg2TiO4 were detected. In the XRD patterns of the samples that were synthesized under other conditions, mixed-phase MgTi2O5/MgTiO3 (MMT) and rhombohedral phase MgTiO3 (JCPDS 06-0494) emerged (as shown in Fig. S1†). The X-ray Energy Dispersive Spectrometer (EDS) results in Fig. 1b show that the sample of PMT contains the three elements Mg, Ti and O. The Raman spectrum in Fig. S2† gives further evidence of MgTi2O5 nanocrystals, as the Raman shifts at 661 cm−1 and 797 cm−1 may be attributed to the vibrations of the O atoms and 159 cm−1, 218 cm−1 and 266 cm−1 may be attributed to the anti-symmetric breathing and twisting vibrations of the O octahedral, according to previous works.17,18
The XPS results in Fig. 2 demonstrate that the Mg and Ti are +2 and +4 without alterable valence states, and the atom ratios of them are also approximately two. According to the results of the XRD, assisted by EDS and XPS, it could be confirmed that pure orthorhombic phase MgTi2O5 was successfully prepared.
The optical absorptions of the pure MgTi2O5 nanocrystals and commercial MgTi2O5 were conducted with a UV-vis absorption spectrometer, as shown in Fig. S3a.† Obviously, both the PMT and CMT show photoabsorption in the UV light region (λ < 400 nm). It should be noted that there are some impurities in the CMT, which is consistent with the results of the XRD (Fig. S3b†). The band gaps (Eg) of the pure MgTi2O5 nanocrystals and commercial MgTi2O5 were calculated to be about 3.45 eV from the onset of the absorption edges.
The morphology of PMT is nanocrystalline with the small size TEM and HRTEM images in Fig. 3a–c showing that the nanocrystals are uniform, well dispersed and the average size is about 20–30 nm. The aggregated nanoparticles form many pores which enhance the surface area and are beneficial to photocatalysis. It is well known that the photocatalytic performance is related to the BET surface area. N2 adsorption–desorption isotherms and corresponding BJH pore size distribution plots of the pure MgTi2O5 nanocrystals and commercial MgTi2O5 were performed, as shown in Fig. S4.† The BET surface areas for the prepared and pure MgTi2O5 nanocrystals, mixed phase MgTi2O5/MgTiO3 and commercial MgTi2O5 are 64.94, 69 and 2.95 m2 g−1, respectively. The HRTEM image clearly shows an interplanar spacing of 0.34 nm, corresponding to the (101) plane of MgTi2O5. The high quality crystallinity and the pure phase are positive to charge transfer and separation.19–21
The as-prepared magnesium titanates are regarded as potential photocatalysts for H2 evolution. Fig. 4a shows the H2 evolution from the prepared pure phase MgTi2O5, mixed-phase MgTi2O5/MgTiO3, and commercial MgTi2O5 under UV light irradiation. It can clearly be seen that the pure phase MgTi2O5 (PMT) displays the highest H2 evolution efficiency, which is up to 418 μmol h−1 (average over three hours), which is much higher than that of the mixed-phase one and the commercial one. It is important that the samples with different surface areas play significant roles, compared to PMT and CMT. However, the surface areas of PMT and MMT-4 are similar, but their efficiencies are very different, which means that there are also other factors affecting the activity. The time dependent H2 evolution curves in Fig. 4b show that the pure phase MgTi2O5 can sustainably produce H2 over time. After recycling five times, the photocatalyst retains its catalytic efficiency, with a decline of about only 10%. This illustrates that the pure phase MgTi2O5 possesses high H2 production efficiency and stability. The crystal structure didn’t change after recycling five times, which can be seen in the XRD pattern in Fig. S5.†
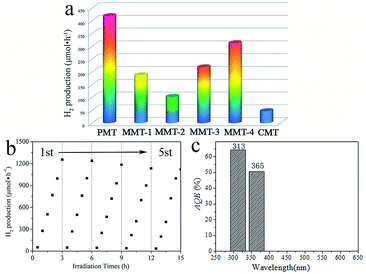 | ||
| Fig. 4 Photocatalytic H2 production of the different samples (a), time dependent H2 evolution and cycle experiment (b) and apparent quantum efficiency (c) of PMT. | ||
In order to demonstrate the relationship between light energy and efficiency, we tested and calculated the apparent quantum efficiency of the MgTi2O5 nanocrystals, which is 62.5% and 50.6% at 313 nm and 365 nm, respectively. This is assessed using the UV-vis absorbance shown in Fig. 4c. This means that the pure phase MgTi2O5 in this work is a UV responsive photocatalyst and UV light is dominant in the photocatalytic efficiency. Significantly, the “solar-to-hydrogen” conversion efficiency (STH) of MgTi2O5 is 0.52%, the computing method for this was followed from a previous report.22
Photoelectrochemical (PEC) performance is a useful technique to demonstrate the charge separation and estimate the water splitting properties.23,24 The PEC properties of PMT were tested, CMT was also tested for contrast. A linear sweep voltammogram of the MgTi2O5 material shows a photocurrent density of 103 μA cm−2, which is almost four times higher than that of the commercial MgTi2O5 material (21 μA cm−2), under UV light irradiation, as shown in Fig. 5a. To further investigate the photocurrent responses of PMT and CMT, transient photocurrents of the samples were carried out during repeated on/off illumination cycles at 0.7 V (vs. Ag/AgCl). Both of the samples showed prompt and reproducible photocurrent responses upon each illumination (Fig. 5b). The transient photocurrent density of PMT is 100 μA cm−2, which is greatly enhanced compared to that of CMT (20 μA cm−2). The transient photocurrent responses match well with the linear sweep voltammograms and display the stability of the magnesium titanate materials.
 | ||
| Fig. 5 Liner sweep voltage (a) and transient photocurrent (b) of pure phase MgTi2O5 (PMT) and commercial MgTi2O5 (CMT). | ||
The photogenerated charge transfer and separation need to overcome the impedance of the interface.25,26 The electrochemical impedance measurements show smaller interfacial resistance for PMT than for CMT, not only in the dark but also under illumination, indicating a more efficient charge separation (Fig. 6a). Mott–Schottky plots could be used to reflect the type of semiconductors, the flat band and the charge density. Fig. 6b demonstrates that both PMT and CMT are n-type semiconductors from the positive slopes. The slope of the plot is inversely proportional to the charge density, the smaller it is, the higher the charge density. PMT showed a substantially smaller slope on the Mott–Schottky plot than CMT, illustrating that PMT possesses higher charge density. Thus, PMT has smaller interface impedance and higher charge density, and also higher photocurrent density. These results give good evidence that PMT is beneficial for charge transfer and separation, possibly due to the pure phase crystal structure and large surface area. Based on the results above, we speculate that the enhancement is caused by (i) the pure phase structure that has the effect of eliminating the harmful impurities of TiO2 or Mg2TiO4 (mismatched electronic band structure); (ii) the large surface area, which is due to the small nanoparticles compared to the commercial sample. It may also be concerned with defects, because many nanomaterials have more or less defects but we have no direct evidence. However, it is believed that this topic of defects in pure phase materials would inspire future interest to focus on the deep mechanism.
Conclusions
In summary, we successfully synthesized pure phase MgTi2O5 nanocrystals via a facile organic ligand coordinated polymerization method that was followed by calcination in air. The pure phase could be controlled by the concentration of metal ions. At a lower concentration, the pure phase could be more easily obtained. The pure phase materials show excellent photocatalytic hydrogen production activity because of the pure phase crystal structure without impurities as well as the large surface area that contributes to charge separation, as demonstrated by PEC. It is believed that the organic ligand coordinated polymerization method could extend to other pure phase titanates or even perovskite photocatalysts for high activity H2 evolution or water splitting.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21171052, 21471050, 21376065, 21501052 and 21473051), the Program for New Century Excellent Talents in University of Ministry of Education of China (NCET-11-0959), the China Postdoctoral Science Foundation. (2015M570304), the Postdoctoral Science Foundation of Heilongjiang Province (LBH-Q11009), Program for Innovative Research Team in University (IRT-1237), Heilongjiang Province Natural Science Foundation (ZD201301, QC2015010) and Harbin Technological Innovation Talent of Special Funds (RC2013QN017028), Youth Science Fund of Heilongjiang University (QL2014).Notes and references
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han and C. Li, Chem. Rev., 2014, 114, 9987–10043 CrossRef CAS PubMed.
- D. Martin, G. Liu, S. Moniz, Y. Bi, A. Beale, J. Ye and J. Tang, Chem. Soc. Rev., 2015, 44, 7808–7828 RSC.
- H. Li, K. Wang, W. Li, S. Cheng and K. Jiang, J. Mater. Chem. A, 2015, 3, 16495–16500 CAS.
- Y. Ide, Y. Nakasato and M. Ogawa, J. Am. Chem. Soc., 2010, 132, 3601–3604 CrossRef CAS PubMed.
- Y. Suzuki and Y. Shinoda, Sci. Technol. Adv. Mater., 2011, 12, 034301 CrossRef.
- K. Surendran, A. Wu, P. Vilarinho and V. Ferreira, Chem. Mater., 2008, 20, 4260–4267 CrossRef CAS.
- O. Ruzimuradov, G. Hasegawa, K. Kanamori and K. Nakanishi, J. Ceram. Soc. Jpn., 2011, 119, 440–444 CrossRef CAS.
- A. Belous, O. Ovchar, D. Durylin, M. Valant, M. Macek-Krzmanc and D. Suvorov, J. Eur. Ceram. Soc., 2007, 27, 2963–2966 CrossRef CAS.
- X. Wang, J. Cai, Y. Zhang, L. Li, L. Jiang and C. Wang, J. Mater. Chem. A, 2015, 3, 11796–11800 CAS.
- R. Hazen and H. Yang, Science, 1997, 277, 1965–1967 CrossRef CAS.
- F. Xie, Y. Deng, Y. Xie, H. Xu and G. Chen, Chem. Commun., 2015, 51, 3545–3548 RSC.
- Y. Suzuki and M. Morimoto, J. Ceram. Soc. Jpn., 2010, 118, 1212–1216 CrossRef CAS.
- F. Wang, Y. Han, C. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463(106), 1–5 Search PubMed.
- L. Wang and Y. Li, Chem. Mater., 2007, 19, 727–734 CrossRef CAS.
- Y. Qu, W. Zhou, K. Pan, C. Tian, Z. Ren, Y. Dong and H. Fu, Phys. Chem. Chem. Phys., 2010, 12, 9205–9512 RSC.
- Y. Qu, W. Zhou, Y. Xie, L. Jiang, J. Wang, G. Tian, Z. Ren, C. Tian and H. Fu, Chem. Commun., 2013, 49, 8510–8512 RSC.
- M. K. Suresh, J. K. Thomas, H. Sreemoolanadhan, C. N. George, A. John, S. Solomon, P. Wariar and J. Koshy, Mater. Res. Bull., 2010, 45, 761–765 CrossRef CAS.
- L. Jing, W. Zhou, G. Tian and H. Fu, Chem. Soc. Rev., 2013, 42, 9509–9549 RSC.
- G. Xiang, T. Li, J. Zhuang and X. Wang, Chem. Commun., 2010, 46, 6801–6803 RSC.
- W. Zhou, W. Li, J. Q. Wang, Y. Qu, Y. Yang, Y. Xie, K. Zhang, L. Wang, H. Fu and D. Zhao, J. Am. Chem. Soc., 2014, 136, 9280–9283 CrossRef CAS PubMed.
- J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S. T. Lee, J. Zhong and Z. Kang, Science, 2015, 6225, 970–974 CrossRef.
- D. Chen, H. Zhang, Y. Liu and J. Li, Energy Environ. Sci., 2013, 6, 1362–1387 CAS.
- M. Zhong, T. Hisatomi, Y. Kuang, J. Zhao, M. Liu, A. Iwase, Q. Jia, H. Nishiyama, T. Minegishi, M. Nakabayash, N. Shibata, R. Niishiro, C. Katayama, H. Shibano, M. Katayama, A. Kudo, T. Yamada and K. Domen, J. Am. Chem. Soc., 2015, 137, 5053–5060 CrossRef CAS.
- T. Butburee, Y. Bai, J. Pan, X. Zong, C. Sun, G. Liu and L. Wang, J. Mater. Chem. A, 2014, 2, 12776–12784 CAS.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Quantum efficiency calculations, Raman, UV-vis, N2 adsorption–desorption isotherm curves and XRD before/after reactions. See DOI: 10.1039/c5ra20992g |
| This journal is © The Royal Society of Chemistry 2015 |