Effect of substrate pre-treatment on microstructure and enhanced electrochromic properties of WO3 nanorod arrays†
Abstract
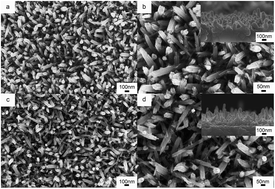
WO3 nanorod arrays (WNRAs) were successfully synthesized on an FTO substrate pre-coated with a layer of TiO2 seeds. The morphology and micro-structure were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), high resolution transmission electron microscopy (HRTEM) and X-ray photoelectron spectroscopy (XPS). The effect of substrate pre-treatment on electrochromic (EC) properties of as-prepared WNRAs was investigated in detail. Compared with those synthesized on WO3 seed layer, the WNRAs grown on TiO2 seed layer showed a large optical modulation (77.5%, at 660 nm) and high coloration efficiency (142.7 cm2 C−1) under the optimal preparation conditions. In addition, the influence of annealing temperature of the TiO2 seed layer on the EC performance was also discussed. More importantly, the WNRAs grown on the TiO2 seed layer presented good stability and strong adhesion to the FTO substrate during the test process.

 Please wait while we load your content...
Please wait while we load your content...