Electrodeposited nickel cobalt sulfide nanosheet arrays on 3D-graphene/Ni foam for high-performance supercapacitors
Abstract
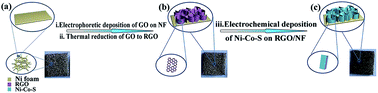
Herein, graphene oxide (GO) with a 3D structure was prepared on the surface of Ni foam (NF) via electrophoretic deposition, and then reacted in situ to form 3D reduced graphene oxide (RGO) via thermal reduction. Ni–Co–S nanosheet arrays were produced on various substrates (RGO/NF, GO/NF and NF) via a facile one-step electrochemical deposition. Scanning electron microscopy (SEM) demonstrated that RGO nanosheets were vertically wrapped on NF by thermal reduction of GO. Furthermore, interconnected and hierarchical porous Ni–Co–S nanosheets were uniformly coated on RGO. The electrochemical performance of the ternary material on different substrates was investigated. The physical structures, combined with the advantages of both ternary Ni–Co–S and RGO, exhibited excellent electrochemical performance. When incorporated as electrode materials for supercapacitors, the synthesized samples possess high specific capacitance and a long cycle life. With the synergistic effect of RGO and ternary Ni–Co–S, a high performance is achieved. The specific capacitance of Ni–Co–S/RGO/NF (2643 F g−1) demonstrated an enhancement compared with Ni–Co–S/GO/NF (2083 F g−1) and Ni–Co–S/NF (1329 F g−1) at a current density of 10 A g−1. Additionally, the retention of specific capacitance of Ni–Co–S/RGO/NF after 2500 cycles displayed a superior cyclic stability of 84.22% at a current density of 50 A g−1.

 Please wait while we load your content...
Please wait while we load your content...