A new and environmentally benign synthesis of aroylguanidines using iron trichloride†
Abstract
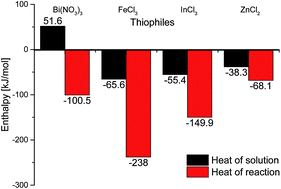
A new synthetic approach for the guanylation of aroylthioureas using iron trichloride is presented. Our synthetic method distinguishes itself by benign reaction conditions, low costs and a broad product spectrum. The scope of the reaction and calorimetric studies are described.

 Please wait while we load your content...
Please wait while we load your content...