Evaluation of in vitro wound adhesion characteristics of composite film and wafer based dressings using texture analysis and FTIR spectroscopy: a chemometrics factor analysis approach†
Abstract
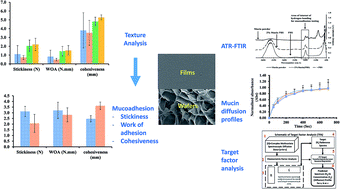
The adhesive properties of two dressing types, solvent cast films and freeze-dried wafers have been determined and compared using two analytical techniques, combined with chemometrics data analysis. Films and wafers were prepared from gels containing polyox (POL) combined with carrageenan (CAR) or sodium alginate (SA), glycerol (GLY) as plasticiser (films) with streptomycin and diclofenac as model drugs. The gels were dried in an oven at 40 °C or freeze-dried to obtain films and wafers respectively. The adhesive performance of the films and wafers was assessed with 6.67% w/v gel using a texture analyser to measure the stickiness, work of adhesion and cohesiveness. The effect of viscosity of simulated wound fluid [containing (2% w/w or 5% w/w bovine serum albumin)] and mucin solution (2% w/w) present on the gelatin surface on texture analyser profiles was investigated. Furthermore, the adhesive properties were estimated and evaluated using attenuated total reflectance Fourier transform infrared spectroscopy by monitoring the diffusion of mucin solution [2% w/w in phosphate buffered saline (PBS) pH 7.4] through the formulations. The diffusion data was analysed using target factor analysis (chemometrics approach) to establish proof of concept for predicting adhesion by measuring mucin interaction and its diffusion through films and wafers. There was a significant effect of simulated wound fluid, viscosity, plasticizer (for films) and drug loading on the adhesive performance of both films and wafers. POL-SA films showed higher mucoadhesive performance in the presence of viscous simulated wound fluid containing 5% bovine serum albumin. Wafers and plasticised films demonstrated high detachment force indicating strong interactions between the chains of the polymers (POL, SA and CAR) and the model wound surface (gelatin). ATR-FTIR spectroscopy showed that mucin diffused independently through the solvent and across the films and wafers. POL-CAR films generally showed slower diffusion of mucin when compared with POL-SA films whilst the opposite effect was observed for diffusion through POL-CAR wafers and POL-SA wafers. Generally, diffusion through wafers was faster than the corresponding films.

 Please wait while we load your content...
Please wait while we load your content...