Superhydrophobic and anti-corrosion Cu microcones/Ni–W alloy coating fabricated by electrochemical approaches
Fengtian Hua,
Penghui Xua,
Haozhe Wangb,
Un byoung Kangc,
Anmin Hu*a and
Ming Li*a
aState Key Laboratory of Metal Matrix Composites, Key Laboratory for Thin Film and Microfabrication Technology of the Ministry of Education, School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, People’s Republic of China. E-mail: huanmin@sjtu.edu.cn; mingli90@sjtu.edu.cn; Tel: +86 021 34202748
bDepartment of Mechanics Engineering, Massachusetts Institute of Technology, USA
cSemiconductor Packaging Development, Samsung Electronics, Korea
First published on 17th November 2015
Abstract
In this work, we present a simple method for fabricating a microstructured Cu/Ni–W alloy coating by combining electroless and electro deposition. Field emission scanning electron microscopy (FESEM) results show that a layer of Ni–W alloy has covered uniformly the conical surface of Cu microcone arrays, forming a multilayer coating. The Tafel curve shows the prominent anti-corrosion property of the as-deposited Ni–W film. Wettability results reveal that the water contact angles can be increased from 106° to 153.2° by adjusting the electrodeposition time of the Ni–W layer. The liquid–solid–air contact mode between the superhydrophobic Ni–W hemisphere decorated Cu microcone array and the water drop is briefly discussed. This work also showed potential for use in a wide range of applications, such as the commercial production of anti-wetting and anti-corrosion devices.
1. Introduction
Wettability is an important feature of a solid surface, which is determined by the balance between adhesive and cohesive forces. This property is usually described by measuring the contact angle, which is formed in the contact interface between the liquid and solid surface. Usually, a surface for which the contact angle is greater than 150 degrees is defined as a superhydrophobic surface. Superhydrophobic surfaces have various functional applications in engineering materials, including the space, construction, automotive, aviation, microelectronics and transportation industries. Research on the surface of engineering materials is of great significance to superhydrophobic surfaces and their application in industry. The wetting properties of the surface of engineering materials determine the adhesion of those materials. Superhydrophobicity possesses a broad prospect for application in self-cleaning,1 fluid drag reduction,2 anticorrosive,3,4 oil–water separation5–7 and so on. Therefore, it has gained the much attention from researchers in recent years.1,8,9Structures at the nanometer and the micrometer scale are required to entrap air below water droplets and thus reduce the liquid–solid contact and cause superhydrophobicity. Jiang et al. reported that the surface of the lotus leaf is covered with dual-scale hierarchical structured protuberances.10 Zhang et al. reported that superhydrophobic nanoporous polydivinylbenzene materials have been synthesized.11 Mo et al. exhibited that self-cleaning and superhydrophobicity can be realized on an Au-coated Ni nanocone array surface.12
As a commonly used material, compared with other metals and alloys, copper and copper alloys havee excellent electrical conductivity, thermal conductivity and electrical resistance transition. As a strategic metal material, it has the extremely widespread application in many fields. In our previous work, Zhang13 successfully fabricated dense copper microcones arrayed structure by electroless plating with crystal modification. However, Cu has a high affinity to oxygen and a soft texture, which leads to inferior device performance and failure. Therefore, one of the biggest challenges of Cu application is the prevention of Cu oxidation and corrosion.14 As an essential engineering material, Ni–W alloy has an excellent high-temperature corrosion resistance and low permeability to diffusion.15–17 When Ni–W alloy coating was deposited on Cu surface, it could protect the Cu surface from corrosion and scratches. The addition of W to the nickel film has attracted a great deal of attention in recent years, especially the Ni–W alloy film.18,19 The W atoms in Ni and their segregation to the grain boundaries make the alloy film more stable. Till now, there are few reports on the surface morphology and superhydrophobic mechanism of Cu microcones/Ni–W alloy coating.
In this work, we have prepared a Cu/Ni–W multilayer coating with micro-posts arrayed on the surface by a facile two-step approach combining electroless with electro deposition. The micro-post morphology after surface modification shows excellent superhydrophobicity. Furthermore, the contact model between the contact angle and the surface morphology is briefly discussed. The synthesis processes of different layers (Cu and Ni–W) are expected to be appropriate for commercial applications.
2. Experiment
A Cu microcone arrays (MCAs)/Ni–W alloy coating was fabricated by a two-step deposition method. Firstly, Cu plates were electrochemically degreased for 1 min, acid-cleaned with 20% HCl for 20 s and PdCl2 activation for 60 s. After the pre-treatment, the copper microcone array was plated on the substrate in the bath solution by electroless deposition. The electrolyte was composed of analytically pure CuSO4·5H2O (0.03 mol L−1), NiSO4·6H2O (0.0024 mol L−1), NaH2PO2·H2O (0.24 mol L−1), Na3C6H5O7·2H2O (0.05 mol L−1) and H3BO3 (0.50 mol L−1) as well as crystallization modified polyethylene glycol (5 ppm), which is referred to as step one. The temperature was kept at 60 °C and the pH value was 8.5–9.0 (adjusted by 20% NaOH solution).Subsequently, a Ni–W (25 wt%) alloy coating was electroplated on the as-prepared copper microcone-arrayed substrate at a constant current density of 10 A dm−2, which is referred to as step two. The electrolyte for electro-plating of the Ni–W alloy coating consisted of analytically pure NiSO4·6H2O (0.22 mol L−1), NaWO4·2H2O (0.07 mol L−1), Na3C6H5O7·2H2O (0.5 mol L−1), NH4Cl (0.50 mol L−1) and NaBr (0.15 mol L−1), as previously reported.20 The deposition time varied from 0.5 min to 2.5 min and the solution temperature was 60 °C. The morphology of the deposits was studied by scanning electronic microscopy (SEM; FEI Sirion 200 HR FE-SEM). X-Ray diffraction (XRD; Rigaku D/MAX-IIIA) was used to identify the phases from 20° to 95° with Cu Kα radiation (λ = 0.15418 nm). Water contact angles (CA) were measured using a sessile drop method of an optical contact angle measuring device (OCA20) at ambient temperature. A drop of 3 μL was placed on the surface, and each reported angle datum is calculated as the average of five measurements in different points on the sample. The electrochemical analysis was performed in a three electrode-system setup on a CHI 660c electrochemical work station at room temperature in 3.5 wt% NaCl solution as the corrosive medium. X-Ray fluorescent spectroscopy (XFS; Fischerscope X-ray XUL-XYm) measurements were carried out to measure the thickness of the Ni–W layer. Each datum in this study was an average of five measurements of different regions in order to achieve more precise data.
3. Results and discussions
3.1 Morphological observation
Fig. 1 demonstrates the typical morphology of a Cu MCAs deposit fabricated by electroless deposition. Cu MCAs are chemically deposited on the copper foil with a size of 2–4 μm in height and about 1 μm in bottom diameter. The copper deposits are fine, dense, and uniform and typically cone shaped. The tips of the MCAs are very sharp. This unique structure is closely related to the concentration and type of crystallization modifier which has been reported in our previous work.21 | ||
| Fig. 1 Low (A) and high (B) magnification SEM images of the copper microcone array structure surface. | ||
Fig. 2 shows the surface morphology of the electroless plated Cu MCAs before and after the deposition of the Ni–W alloy coating with different deposition times. Fig. 2(A) illustrates the typical cone structure. Fig. 2(B)–(F) reveal the structure evolution corresponding to different deposition times. With increasing the Ni–W alloy deposition time, the tips of theCu MCAs become less sharp, and the microcones are gradually transformed to a hemispherical structure. This trend is extremely obvious after the deposition time reaches 1.5 min (Fig. 2(D)), from which we can see that the gaps among the Cu MCAs are filled up and the surface becomes smooth. As the Ni–W alloy deposition layer becomes thicker, the original Cu MCAs disappear totally and the surface is finally coated with tightly packed micro-hemispheres, as shown in Fig. 2(F).
 | ||
| Fig. 2 SEM images of Cu MCAs surface after deposition of Ni–W alloy coating for (A) 0 min, (B) 0.5 min, (C) 1 min, (D) 1.5 min, (E) 2 min and (F) 2.5 min. | ||
3.2 Influence of deposition time on thickness
The relationship between the thickness of the Ni–W alloy layer and plating time is illustrated in Fig. 3. Under each condition, 5 specimens were tested for statistical accuracy. When specimens were plated for 0.5 min, 1 min, 1.5 min, 2 min and 2.5 min, the average thicknesses of the five conditions was measured to be 0.73 μm, 1.43 μm, 2.15 μm, 2.88 μm and 3.59 μm, respectively. With increasing deposition time, the thickness of the Ni–W coating increases. The linear relationship between the thickness of the Ni–W layer and the deposition time accords with the following equation:| d = 1.43t | (1) |
3.3 XRD analysis
Fig. 4 shows the X-ray diffraction pattern of the Cu MCAs/Ni–W (25 wt%) alloy coating, demonstrating the existence of both Cu and Ni–W alloy. Three strong diffraction peaks near the diffraction angles of 43°, 50° and 74° are in accordance with diffractions of Cu crystal faces (111), (200) and (220), respectively, which can be indexed as face-centered cubic (fcc) Cu (JCPDS file no. 040836), as shown Fig. 4(A). It is clearly seen from Fig. 4(B) that three peaks appear near 44.3°, 51.8° and 75.9°, indicating the formation of a crystal structure. It is well acknowledged that three diffraction peaks of pure W are 2θ1 = 40.26°, 2θ2 = 58.36°, 2θ3 = 73.38° and that of pure Ni are 2θ1 = 44.62°, 2θ2 = 51.94°, 2θ3 = 76.14°, corresponding to the diffractions of Ni crystal faces (111), (200) and (220), which can be indexed as fcc Ni (JCPDS file no. 040850). This result shows that the peak position and peak intensity of the Ni–W alloy and pure Ni are extremely close, demonstrating that the alloy is a substitutional solid solution, in which Ni is the solvent and W is the solute. Since the radius of an W atom is larger than that of an Ni atom, the Ni lattice expansion is caused by the formation of a solid solution. This makes the interplanar spacing increase and 2θ moves in the lower direction according the Prague formula, which agrees with the experimental results.3.4 Microhardness
The Vickers hardness of pure Cu is 369 MPa far lower than that of Ni and W, while the Vickers hardness of pure Ni and W are 638 MPa and 3430 MPa, respectively. Therefore, it can be inferred that the hardness value of the Ni–W film on the surface of Cu MCAs increases with the increase of the thickness of the Ni–W film and the overall hardness value of the substrate is increased.22 The surface hardness of the substrate was measured with a Vickers hardness tester. With increasing deposition time, the hardness value of surface increases because of the increase of the thickness of Ni–W alloy, as shown in Fig. 5. This can improve the texture of copper and prevent scratching.3.5 Anti-corrosion properties
A Tafel curve is established to explain the anti-corrosion properties. In the Tafel region of the electrode curve, the relationship between thecorrosion potential and the corrosion current density can be described in terms of the equation:
E = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[I] log[I]
| (2) |
3.6 Superhydrophobicity and weather-resistance
The static contact angle was measured to evaluate the wettability of the Ni–W alloy coating. As shown in Fig. 7, the Cu MCAs surface shows a contact angle of 106°. The hydrophobicity of the coating deposited with Ni–W alloy shows a highest contact angle of 153.2°. However, as the deposition of Ni–W alloy continues, the water contact angle of the surface is reduced instead. In order to evaluate the effect of various corrosive solutions on the wettability of the Ni–W alloy coating (deposition time is 1.5 min), the water contact angles after immersion in solutions with different pH values for 2 h were measured (Fig. 8). The contact angle is larger than 150° when the pH is 1 or 14. Other conditions, like freezing for 2 h, heating to 200 °C for 2 h and leaving for 2 months (Fig. 8), were also shown to have little influence on the superhydrophobicity. | ||
| Fig. 7 Optical graphs of water droplets (3 μL) on the Cu MCAs/Ni–W alloy coating surface corresponding to the deposition time of Ni–W alloy. | ||
Combining the above results, it can be concluded that the superhydrophobicity of the resulting Cu MCAs surface must correlate closely with the surface roughness. Liquids are assumed to contact only the sharp tips of the cones and air pockets are trapped beneath the liquid, which gives a composite state. In this state, the air parts of the surface can be considered completely non-wetting. According to Cassie’s equation, the smaller area fraction of the vapor on the surface will lead to a decrease of the contact angle.24,25 Therefore, with the increase of the Ni–W alloy deposition time, the air volume beneath the water droplet decreases, which leads to a decrease of the contact angle. According to Nosonovsky and Bhushan’s theory,26 a hemispherical top is more desirable for a maximum contact angle than cones since sharp edges may lead to pinning of the triple line and the grooves may act as open capillaries to reinforce wetting. Besides, the spaces among the micro-hemispheres can hold more air pockets thus preventing the droplet from touching the valley between the microcones. Fig. 9 illustrates the contact modes of water droplets on two different micro-structured surfaces. This prominent property of films can be applied to self-cleaning and corrosion prevention.
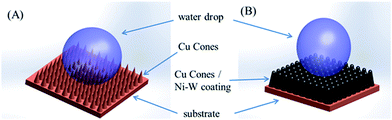 | ||
| Fig. 9 Schematics of two different wetting modes on (A) Cu MCAs and (B) Cu MCAs/Ni–W micro-post arrayed structures. | ||
4. Conclusions
In summary, the Cu MCAs/Ni–W alloy coating with a hemispherical top is fabricated by the employment of electroless and electro deposition. With different deposition times, the morphology can be modified and the related growth mechanism is studied. It shows superhydrophobicity with a water contact angle of 153.2°. The hemispherical top of the micro-post avoids the pinning of the triple line and plays an important role in superhydrophobicity. The Tafel curve shows that the Cu MCAs coated Ni–W alloy film has excellent anti-corrosion properties. This research may enrich our understanding of the metallic materials and microstructures, which may have broad prospects in fields like the fabrication of anti-wetting materials.Acknowledgements
This work is sponsored by the National Natural Science foundation of China (61176097, 61376107) and the National Basic Research Program of China (973 Program, 2015CB057200). We also thank the Instrumental Analysis Center of Shanghai Jiao Tong University for the use of the SEM/EDS equipment.References
- Y.-L. Zhang, H. Xia, E. Kim and H.-B. Sun, Soft Matter, 2012, 8, 11217–11231 RSC.
- F. Shi, J. Niu, J. Liu, F. Liu, Z. Wang, X. Q. Feng and X. Zhang, Adv. Mater., 2007, 19, 2257–2261 CrossRef CAS.
- F. Zhang, L. Zhao, H. Chen, S. Xu, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2008, 47, 2466–2469 CrossRef CAS PubMed.
- T. Liu, Y. Yin, S. Chen, X. Chang and S. Cheng, Electrochim. Acta, 2007, 52, 3709–3713 CrossRef CAS.
- L. Feng, Z. Zhang, Z. Mai, Y. Ma, B. Liu, L. Jiang and D. Zhu, Angew. Chem., 2004, 116, 2046–2048 CrossRef.
- Q. Wang, Z. Cui, Y. Xiao and Q. Chen, Appl. Surf. Sci., 2007, 253, 9054–9060 CrossRef CAS.
- M. Wang and H. Wang, Appl. Surf. Sci., 2008, 254, 6002–6006 CrossRef.
- J. Drelich, E. Chibowski, D. D. Meng and K. Terpilowski, Soft Matter, 2011, 7, 9804–9828 RSC.
- R. Ramachandran and M. Nosonovsky, Soft Matter, 2014, 10, 7797–7803 RSC.
- X. Feng and L. Jiang, Adv. Mater., 2006, 18, 3063–3078 CrossRef CAS.
- Y. Zhang, S. Wei, F. Liu, Y. Du, S. Liu, Y. Ji, T. Yokoi, T. Tatsumi and F.-S. Xiao, Nano Today, 2009, 4, 135–142 CrossRef CAS.
- X. Mo, Y. Wu, J. Zhang, T. Hang and M. Li, Langmuir, 2015, 31, 10850–10858 CrossRef CAS.
- W. Zhang, Z. Yu, Z. Chen and M. Li, Mater. Lett., 2012, 67, 327–330 CrossRef CAS.
- R. Berriche, R. Lowry and M. I. Rosenfield, An Oxidation Study of Cu Leadframes, 1999 Search PubMed.
- T. Yamasaki, R. Tomohira, Y. Ogino, P. Schlossmacher and K. Ehrlich, Plat. Surf. Finish., 2000, 87, 148–152 CAS.
- Y. Shacham-Diamand and Y. Sverdlov, Microelectron. Eng., 2000, 50, 525–531 CrossRef CAS.
- W.-H. Hui, J.-J. Liu and Y.-S. Chaug, Surf. Coat. Technol., 1994, 68–69, 546–551 CrossRef.
- H. R. Kotadia, O. Mokhtari, M. Bottrill, M. P. Clode, M. A. Green and S. H. Mannan, J. Electron. Mater., 2010, 39, 2720–2731 CrossRef CAS.
- K. Sriraman, S. G. S. Raman and S. Seshadri, Mater. Sci. Eng., A, 2006, 418, 303–311 CrossRef.
- F. Hu, H. Wang, S. Yang, A. Hu and M. Li, Appl. Surf. Sci., 2015, 353, 774–780 CrossRef CAS.
- W. Zhang, X. Feng, H. Cao, A. Hu and M. Li, Appl. Surf. Sci., 2012, 258, 8814–8818 CrossRef CAS.
- T. Yamasaki, Scr. Mater., 2001, 44, 1497–1502 CrossRef CAS.
- M. Obradovic, J. Stevanovic, A. Despic, R. Stevanoyic and J. Stoch, J. Serb. Chem. Soc., 2001, 66, 899–912 CAS.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988–994 CrossRef CAS.
- A. Cassie, Discuss. Faraday Soc., 1948, 3, 11–16 RSC.
- M. Nosonovsky and B. Bhushan, J. Phys.: Condens. Matter, 2008, 20, 225009–225038 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |





