Photochemical upconversion in metal-based octaethyl porphyrin–diphenylanthracene systems†
Abstract
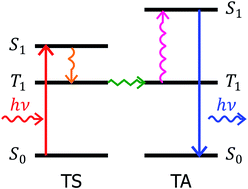
This paper studies photochemical upconversion in solutions of octaethyl porphyrin (OEP) and diphenyl anthracene (DPA). The system has been widely used as a standard model system in the field of photochemical upconversion. Although, the kinetics of elementary processes contributing to photochemical upconversion in it have been extensively studied, there has been no research on the efficiency of upconversion in the system, despite of the fact that this parameter is detrimental for potential applications of photochemical upconversion process. We determine the yield of photochemical upconversion in a number of metal based OEP/DPA systems. Additionally, we studied the dependence of kinetic, and efficiency parameters of the process on the core metal of the porphyrin. We showed that the overall efficiency of photochemical upconversion depends significantly on the core metal of the triplet sensitizer porphyrin molecule. We attribute this effect to the differences in efficiency of triplet energy transfer from metal based OEP to DPA depending on the core metal.

 Please wait while we load your content...
Please wait while we load your content...