Alginate surfactant derivatives as an ecofriendly corrosion inhibitor for carbon steel in acidic environments†
Abstract
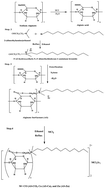
Biopolymer alginate surfactant derivatives were synthesized and their influences as a novel corrosion inhibitor on carbon steel in 1 M HCl were studied using gravimetric, electrochemical, EDX and SEM techniques. The compounds obtained were characterized using FTIR, 1H NMR and UV-vis spectroscopy studies. The inhibition efficiency increased with the increase in concentration and reached a maximum of 96.27% for AS–Cu at 5 × 10−3 M concentration. Potentiodynamic polarization results reveal that alginate derivatives could be classified as mixed-type corrosion inhibitors with predominant control of the cathodic reaction. The extent of inhibition exhibits a positive trend with an increase in temperature. The Langmuir isotherm provides the best description of the adsorption nature of the inhibitor. The results of EIS indicate that both the charge transfer resistance and inhibition efficiency tend to increase by increasing the inhibitor concentration. The thermodynamic parameter and activation parameters were calculated to investigate the mechanism of inhibition. Also, the relationship between the chemical structure and inhibition efficiency of the inhibitor was discussed.

 Please wait while we load your content...
Please wait while we load your content...