Optimization and hydrolysis of cellulose under subcritical water treatment for the production of total reducing sugars†
Abstract
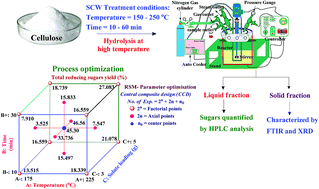
Subcritical water (SCW) treatment has gained enormous attention as an environmentally friendly technique for organic matter and an attractive reaction medium for a variety of applications. In this work, hydrolysis of cellulose was studied under SCW conditions in a batch reactor to attain total reducing sugars (TRS) within a reaction temperature and time range of 150 to 250 °C and 10–60 min, respectively. From the experimental results, the highest yield of TRS was 45.04% as obtained at 200 °C and 20 min of hydrolysis time. The characterisation techniques, namely X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy were used as to determine the structural and compositional changes in the hydrolysed material. Reaction parameters such as temperature, time, and solute loading have been optimised using response surface methodology based on a central composite design. From ANOVA analysis, it was described that the second-order response surface model is highly significant as per Fisher's F-test and P-value. A first-order reaction kinetic model was formulated to describe the hydrolysis of cellulose for TRS formation and decomposition. For TRS formation, the activation energy and pre-exponential factor of the Arrhenius equation was found to be 29.16 kJ mol−1 and 0.088 min−1 for 60 min, respectively.

 Please wait while we load your content...
Please wait while we load your content...