Electrochemical Ostwald ripening and surface diffusion in the galvanic displacement reaction: control over particle growth†
Abstract
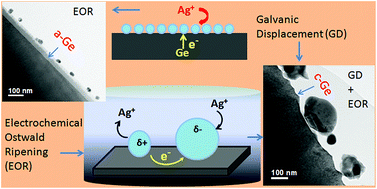
We report the role of electrochemical Ostwald ripening and the galvanic displacement reaction in uniform particle formation on ion bombarded amorphous (i.e., composed of random atomic spacings) Ge (a-Ge) surfaces compared to crystalline germanium (c-Ge). Silver growth on c-Ge and a-Ge substrates by electroless deposition has been studied by atomic force microscopy (AFM), cross-sectional transmission electron microscopy (XTEM), and energy dispersive X-ray spectroscopy (EDX). Introduction of defects can provide a control over particle growth by the galvanic displacement reaction that may offer continuous film growth up to a definite thickness. The possibility of surface diffusion and its consequences have also been discussed.
- This article is part of the themed collection: Nanoscience and nanotechnology in electrochemistry


 Please wait while we load your content...
Please wait while we load your content...