Silicon-supported aluminum oxide membranes with ultrahigh aspect ratio nanopores†
Abstract
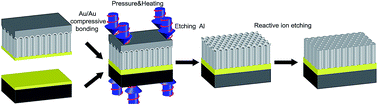
AAO membranes become essential for fabricating nano-building blocks. However, the integration of these nano-building blocks in complex machinery is still challenging, mainly due to the fragility of these membranes. In this work, we overcome this drawback by developing a new integrative process which enables the support of a highly-ordered nanoporous membrane onto a mechanically robust substrate such as silicon. The fabrication of supported AAO (SAAO) membranes is achieved by transferring an AAO layer onto a Si substrate via a Au/Au compressive bonding process. Two types of AAOs were prepared for this bonding process to demonstrate the universality of our technology: mild-anodized AAO (MA-AAO) and pulse-anodized AAO (PA-AAO). We also demonstrate that the newly developed SAAO membranes are suitable for electrodeposition of nanostructures. Problems such as membrane handling or electrolyte leakage occurring in conventional AAO membranes are avoided, so that Ni nanostructures with well-controlled dimensions and uniform lengths are obtained. The high-aspect ratio Ni nanostructures have the potential to be used in various applications, such as biosensing and energy storage.

 Please wait while we load your content...
Please wait while we load your content...