A two-step method for preparing Li4Ti5O12–graphene as an anode material for lithium-ion hybrid capacitors
Abstract
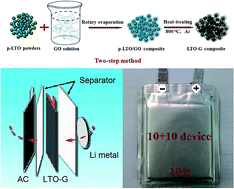
Lithium-ion hybrid capacitors (LICs) are expected to fill the gap between lithium-ion batteries and electrochemical supercapacitors. In this paper, we synthesize Li4Ti5O12–graphene (LTO–G) by a two-step method and use it as the anode material in AC/LTO–G Li-ion hybrid capacitors. The LTO–G composite prepared by the two-step method shows the best electrochemical properties in various ways, and delivers a high specific capacity of 194 mA h g−1 at 0.1C, and 90 mA h g−1 at 28.6C. The AC/LTO–G capacitors can perform well between 1 and 2.5 V, and they deliver a reversible capacity of 80 mA h g−1 at 0.1C. The energy density based on the total active material for these capacitors is 15 W h kg−1 at 4000 W kg−1, and 30 W h kg−1 at 1000 W kg−1. After 10 000 cycles, these capacitors still deliver an energy density higher than 22 W h kg−1 at 1000 W kg−1. The highest energy density based the total mass of the device is 6.6 W h kg−1 and there is still much room for improvement, indicating that the LTO–G composite is a promising candidate anode material for Li-ion hybrid capacitors.

 Please wait while we load your content...
Please wait while we load your content...