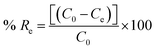Determination of losartan potassium in the presence of hydrochlorothiazide via a combination of magnetic solid phase extraction and fluorometry techniques in urine samples
Amir Farnoudian-Habibia,
Sahar Kangarib,
Bakhshali Massoumic and
Mehdi Jaymand*a
aResearch Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, P.O. Box: 51656-65811, Tabriz, Islamic Republic of Iran. E-mail: m_jaymand@yahoo.com; m.jaymand@gmail.com; jaymandm@tbzmed.ac.ir; Fax: +98-41-33367929; Tel: +98-41-33367914
bDepartment of Chemistry, Science and Research Branch, Islamic Azad University, P.O. Box: 14515-775, Tehran, Islamic Republic of Iran
cDepartment of Chemistry, Payame Noor University, P.O. BOX: 19395-3697, Tehran, Islamic Republic of Iran
First published on 20th November 2015
Abstract
A sensitive and highly efficient method for the determination of losartan potassium (LOS) in the presence of hydrochlorothiazide (HCTZ) via a combination of magnetic solid phase extraction (MSPE) and fluorometry techniques is suggested. For this purpose, imidazolium ionic liquid (Imz)-modified Fe3O4@SiO2 nanoparticles (Fe3O4@SiO2-Imz) were utilized as an adsorbent for the MSPE. Fe3O4@SiO2-Imz exhibited excellent extraction performance for LOS, in part due to the anion-exchange groups in the Imz moiety. The experimental parameters, such as sample pH values, the amount of adsorbent, the extraction time, the desorption solvent, and the desorption time, that could affect the extraction performance were examined and optimized. The determination of LOS in the presence of HCTZ in urine samples was carried out using selective and sensitive excitation–emission fluorescence spectroscopy (EEFS), because only LOS exhibited fluorescence emission at 325 nm, while the HCTZ did not exhibit any fluorescence emission. The limit of detection (LOD) for LOS using this technique was 0.12 ng ml−1.
1. Introduction
Losartan potassium salt (2-n-butyl-4-chloro-5-hydroxymethyl-1-[(2%-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]imidazole) is a prototype of a new generation of effective and orally active non-peptide angiotensin II receptor antagonists. These substances have been developed in sequence with angiotensin converting enzyme inhibitors as a further therapeutic action on the renin–angiotensin–aldosterone system, one of the most important regulators of blood pressure.1,2 It is well established that the 5-carboxylic acid metabolite of losartan is a potent angiotensin II antagonist.3–5 Hydrochlorothiazide (HCTZ), or 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide, is a diuretic of the class of benzothiadiazines widely used in antihypertensive pharmaceutical formulations, alone or in combination with other drugs, which decrease active sodium re-absorption, and reduce peripheral vascular resistance. These two mentioned drugs (LOS and HCTZ) are commonly used in association in the treatment of hypertension.6,7 The chemical structures of these drugs are shown in Scheme 1.Several analytical methods such as high-performance liquid chromatography (HPLC),8,9 reversed phase HPLC (RP-HPLC),10,11 LC-MS,12,13 colorimetery,14 complex formation,15,16 charge-transfer complex formation,17 and capillary electrophoresis (CE)18 have been applied to the determination of LOS in the presence of HCTZ. On the other hand, in the last decade a great deal of research efforts have been focused on the development of new and efficient methods for the extraction, concentration, and isolation of real samples to enhance the sensitivity and selectivity of analytical methods. In this regard, solid phase extraction (SPE) can be considered as an efficient and versatile approach, in part due to its improvements in automation, reproducibility, and high-throughput capability.19–21
However, the request for new adsorbents is an important factor in improving analytical sensitivity, and precision in SPE techniques. To date, many adsorbents, such as active carbon,22 modified resin,23 fullerenes,24 and conductive polymers,25,26 have been employed in SPE. A relatively novel achievement in the field of SPE is the use of magnetic nanoparticles (MNPs), which leads to so-called magnetic solid phase extraction (MSPE). Some advantages of the MSPE method over SPE are its excellent adsorption efficiency and rapid separation from the matrix through an external magnetic field without retaining residual magnetization after its removal, and MSPE has recently exhibited significant advantages in separation science.27–29
This paper describes a novel, sensitive, and selective method for the determination of losartan potassium (LOS) in the presence of hydrochlorothiazide (HCTZ) in human urine samples. According to the drug loading process (as described in the Experimental section), it was found that HCTZ had an interfering role in this study, due to its low loading on the adsorbent surface, and its lack of exhibition of fluorescence emission. Hence, we optimized all parameters, such as pH values, the amount of adsorbent, the extraction time, the desorption solvent, and the desorption time, for MSPE and the determination of LOS.
2. Experimental
2.1. Material
All chemicals including methanol, acetonitrile, FeCl3·6H2O (99%), FeCl2·4H2O (98%), tetraethylorthosilicate (TEOS) (98%), and 3-chloropropyltrimethoxysilane (CPTMS) were of analytical reagent grade or the highest purity available from Merck (Darmstadt, Germany). Double distilled water (DDW) was utilized throughout the study. Standard solutions of LOS and HCTZ were prepared daily by dissolving 0.1 mg of each in 100 ml of DDW. The 0.1 mol l−1 solution of NaOH for pH adjustment was obtained through the dilution of a Merck standard solution of NaOH. All of the solutions were prepared daily, and drug solutions were kept at 5 °C.2.2. Synthesis of Fe3O4@SiO2 core–shell nanoparticles
Magnetite NPs were prepared using the chemical co-precipitation method under alkaline conditions.30 In order, the molar ratio of Fe2+ to Fe3+ salts was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. In a typical synthesis, FeCl3·6H2O (5.4 g, 20 mmol) and FeCl2·4H2O (1.95 g, 10 mmol) were dissolved in DDW (50 ml) with vigorous stirring. The solution was deoxygenated by bubbling highly pure argon gas through the solution for 20 minutes, and then was heated to 80 °C. After vigorous stirring for about 1 hour, 20 ml of NH4OH (28 wt%) was added dropwise. The resultant suspension was maintained at 80 °C for another 1 hour with vigorous stirring, and was then cooled to room temperature. The black powder was collected using an external magnetic field, washed with 50% aqueous ethanol several times, and dried under vacuum at 80 °C.
2. In a typical synthesis, FeCl3·6H2O (5.4 g, 20 mmol) and FeCl2·4H2O (1.95 g, 10 mmol) were dissolved in DDW (50 ml) with vigorous stirring. The solution was deoxygenated by bubbling highly pure argon gas through the solution for 20 minutes, and then was heated to 80 °C. After vigorous stirring for about 1 hour, 20 ml of NH4OH (28 wt%) was added dropwise. The resultant suspension was maintained at 80 °C for another 1 hour with vigorous stirring, and was then cooled to room temperature. The black powder was collected using an external magnetic field, washed with 50% aqueous ethanol several times, and dried under vacuum at 80 °C.
In order to obtain well-dispersed Fe3O4 NPs, the prepared MNPs were added to citric acid (10 ml, 0.1 mol l−1) and sonicated for 30 minutes. The reaction was maintained for 12 hours at room temperature with vigorous stirring, and at the end of this time, the product was washed several times using DDW. The obtained citrate-coated MNPs (CMNPs) were separated with an external magnetic field. It is important to note that citric acid was used as a coating agent for the colloidal stabilization of the MNPs in aqueous solution.
The Fe3O4@SiO2 core–shell NPs were prepared by the growth of a silica layer on Fe3O4 NPs. Therefore, the obtained CMNPs (1.0 g) were added to 10% aqueous ethanol solution (20 ml), and the mixture was sonicated for 20 minutes. Under continuous stirring, ammonia solution (28 wt%; 5 ml) and tetraethoxyorthosilicate (TEOS, 5 ml) were added to the reaction mixture. The resulting mixture was stirred under a N2 atmosphere for 12 hours at room temperature. Then, the core–shell NPs were separated using an external magnetic field to eliminate the homogeneous silica nuclei. The product was washed several times with water and ethanol, and dried overnight under vacuum at room temperature.
2.3. Synthesis of Fe3O4@SiO2-Imz
The obtained Fe3O4@SiO2 core–shell NPs (1 g) were dispersed in a methanol (50 ml) and toluene (5 ml) mixture under sonication for about 20 minutes, and then, 3-chloropropyltrimethoxysilane (CPTMS, 2 ml) was added dropwise to the reaction mixture. The reaction mixture was stirred for about 48 hours under an argon atmosphere, and then centrifuged, washed several times with methanol, and dried overnight under vacuum at room temperature. Afterward, the CPTMS-modified Fe3O4@SiO2 nanoparticles (0.6 g) were added to a solution of imidazole (0.7 g, 10 mmol) in CHCl3 (50 ml), and the suspension was refluxed for 24 hours at 50 °C. The obtained brownish nanoparticles were washed using CHCl3 (3 × 20 ml), and dried under vacuum at room temperature.312.4. Solid phase extraction and preconcentration
Briefly, the Fe3O4@SiO2-Imz nanoparticles (1 mg) were activated with 10 ml of NaOH solution (0.01 mol l−1) for about 12 hours, and then washed with DDW several times. Then, 250 ml (0.1 mg l−1) of LOS–HCTZ mixture (the pH was adjusted to 8 using 0.1 mol l−1 NaOH solution) was added. After vigorous stirring for about 10 minutes, the adsorbent was collected using an external magnetic field. Spectrophotometric results indicated that the loading efficiency was 99% and 44% for LOS and HCTZ, respectively. The adsorption percent (% Re) was determined using the following equation:where C0 and Ce represent the initial and final (after adsorption) concentration of the species, respectively. After discarding the supernatant, 2 ml of desorption solution (a mixture of methanol and NaOH (20% w/v)) was added to the system, and stirred for about 15 minutes to desorb the loaded drugs. After the desorption process, the solution was decanted, and injected into the fluorometry equipment for more analysis.
2.5. Instrumentation
The magnetic properties of the samples were investigated using a vibrating-sample magnetometer (VSM, AGFM, Iran) at room temperature. Scanning electron microscopy (SEM) images were collected using an LE 440I SEM (Oxford, UK) scanning electron microscope. The samples for SEM imaging were coated with a thin layer of gold film to avoid charging. X-ray diffraction (XRD) spectra were obtained with a Siemens D 5000 (Aubrey, Texas, USA), X-ray generator (CuKα radiation with λ = 1.5406 Å) with a 2θ scan range of 2 to 80° at room temperature. Fluorescence spectra were recorded using a FP-6200 spectrofluorometer (JASCO Corporation, Tokyo, Japan) with a wavelength range of 100–400 nm (with 1 nm intervals) for excitation. The instrument was equipped with a 150 W xenon lamp and dual monochromators (a silicon photodiode for excitation and a photomultiplier for emission). The slit widths for both excitation and emission were set at 5 nm, and the fluorescence spectra were recorded at a scan rate of 250 nm min−1.3. Results and discussion
3.1. Characterization of Fe3O4@SiO2-Imz
The X-ray diffraction pattern of the Fe3O4@SiO2-Imz nanoparticles is shown in Fig. 1. All of the observed diffraction peaks are indexed using the cubic structure of the Fe3O4 nanoparticles. As can be seen, the XRD pattern of the Fe3O4@SiO2-Imz nanoparticles is in agreement with the standard Fe3O4 structure, and indicated that these particles have phase stability, and also that the structural integrity was preserved. The diffraction peaks at 2θ = 29.7, 35.5, 43.2, 53.2, 57.1, 62.2, and 74.3° correspond to the (220), (311), (400), (422), (511), (440), and (533) planes of the Fe3O4 crystalline structure, respectively. In addition, a broad diffraction peak in the range of 2θ = 5 to 20° may be attributed to the amorphous silica layer.To obtain a better visual insight into the morphology of the synthesized Fe3O4@SiO2-Imz nanoparticles, a scanning electron microscopy (SEM) image was obtained. Fig. 2 shows the SEM image of the Fe3O4@SiO2-Imz nanoparticles. As seen in this image the average diameter of the nanoparticles is ∼20 nm.
The magnetic properties of the Fe3O4@SiO2-Imz nanoparticles were characterized by means of a vibrating sample magnetometer (VSM). A typical room temperature magnetization curve of Fe3O4@SiO2-Imz is shown in Fig. 3. According to this figure, the magnetic saturation value of the Fe3O4@SiO2-Imz nanoparticles is 46 emu g−1.
3.2. Optimization of MSPE process
In order to achieve high extraction and preconcentration efficiency, the factors affecting the adsorption and desorption processes, such as the amount of adsorbent, eluent type, desorption time, pH value, and sample volume, were optimized. The results obtained are discussed in the following sections.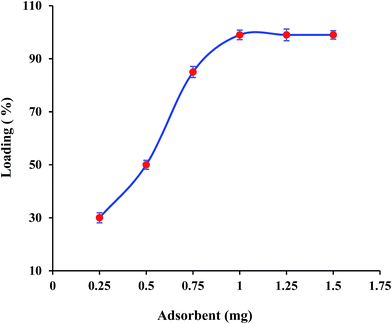 | ||
| Fig. 5 The effect of adsorbent amount on the loading efficiency of LOS [adsorbent (mg)/loading efficiency (%)/RSD (%): 0.25/30/6.33; 0.50/50/3.40; 0.75/85/2.47; 1/99/1.81; 1.25/99/2.22; 1.5/99/1.67]. | ||
3.3. Equilibrium adsorption study
The adsorption isotherm is defined as the relationship between the amount of a substance adsorbed per unit mass of an adsorbent at a given temperature, and its concentration in the equilibrium solution. It is well established that the adsorption isotherm is an essential way to describe how solutes interact with the sorbent. Developing an appropriate isotherm model for adsorption is essential to the design and optimization of adsorption processes. In this respect several isotherm models, such as the Langmuir, Freundlich, Redlich–Peterson, Dubinin–Radushkevich, Sips, and Temkin models, have been developed for evaluating the equilibrium adsorption of compounds from solutions.32 However, the most commonly used model to investigate the adsorption isotherm is the Langmuir equation. Thus, the experimental results of this study were fitted with this model. The equilibrium adsorption isotherm is important in determining the adsorption capacity of LOS and diagnoses the nature of adsorption onto the Fe3O4@SiO2-Imz nanoparticles. The equilibrium adsorption capacity of the adsorbent was calculated using the following equation:where qe is the equilibrium adsorption capacity (mg g−1), C0 is the initial concentration of the LOS (mg l−1), Ce is the equilibrium concentration of LOS (mg l−1), V is the volume of LOS solution (l), and W is the weight of the adsorbent (g). The equilibrium adsorption of LOS solutions by the adsorbent was measured (C0 of LOS = 0, 5, 10, 15, 20, 50, 100, 200, and 300 mg l−1) after the equilibrium time. The well known Langmuir equation, which is valid for monolayer sorption on a surface with a finite number of identical sites, is given by the following equation:
where qmax is the maximum adsorption at the monolayer (mg g−1), and Kads is the Langmuir constant related to the affinity of the binding sites (mg l−1) (Fig. 7).33 A linearized plot of Ce/qe against Ce gives qmax and Kads. The essential characteristics of the Langmuir isotherm can also be expressed in terms of a dimensionless constant of the separation factor or equilibrium parameter (RL) which is defined as follows:
where b is the Langmuir constant. The RL value indicates the shape of the isotherm.34 RL values between 0 and 1 indicate favorable adsorption, while RL ≥ 1, and RL = 0 indicate unfavorable, linear, and irreversible adsorption isotherms. The results obtained from the equilibrium adsorption study are summarized in Table 1.
| Sample | qmax (mg g−1) | b (KLqmax−1) | KL (l mg−1) | r |
|---|---|---|---|---|
| Losartan | 260 | 0.370 | 73.90 | 0.992 |
3.4. Desorption and preconcentration
Various parameters, such as the type and volume of the solvent, volume of the sample, and desorption time, that could affect the drug desorption efficiency from the Fe3O4@SiO2-Imz nanoparticles were studied and optimized in the following sections.| Solvent | Desorption efficiency (%) |
|---|---|
| Acetonitrile | 92 |
| Methanol | 95 |
| Ethanol | 85 |
| Dioxane | 60 |
| n-Hexane | 50 |
| Methanol/NaOH (20% w/v) | 98 |
3.5. Determination of losartan
As is known, LOS shows fluorescence emission at 325 nm (excited at 250 nm), whereas we find that the HCTZ did not exhibit any fluorescence emission. This matter is the basis of the selective determination of LOS in the presence of HCTZ. It should be pointed out that we recorded the excitation–emission fluorescence spectra of the mixture of LOS/HCTZ solution, and only the emission of LOS without any decrease in intensity or shift in the emission maximum was observed. Fig. 11 shows the excitation–emission fluorescence (λexc = 250 nm, λemi = 325 nm) spectra of the LOS. | ||
| Fig. 11 The excitation (a), and emission (b) fluorescence (λexc = 250 nm, λemi = 325 nm) spectra of the LOS. | ||
| LOD = kSDa/b |
In order to evaluate the analytical applicability of the proposed method in the absence and presence of HCTZ in human urine samples, the accuracy and precision were examined. The urine samples were analyzed after spiking with five different concentrations of LOS. The results obtained are summarized in Table 4. As seen in this table, good recoveries were obtained from the procedure, and the presence of HCTZ had no considerable effect on the determination of LOS. In addition, the performance of the designed method in comparison with other techniques is presented in Table 5.
| Spiked value of LOS (ng ml−1) | Spiked value of HCTZ (ng ml−1) | Found value (ng ml−1) | Intraday-RSD (%) (n = 5) | Interday-RSD (%) (n = 5) | Recovery of LOS (%) |
|---|---|---|---|---|---|
| 1 | 1 | 1.10 | 6.5 | 6.8 | 110 |
| 5 | — | 4.80 | 2.5 | 4.5 | 96 |
| 10 | 5 | 10.10 | 2.3 | 4.1 | 101 |
| 25 | — | 24.80 | 0.9 | 1.6 | 99.2 |
| 50 | 30 | 49.70 | 0.5 | 1.2 | 99.4 |
| Determination method | LOD | Reference |
|---|---|---|
| Suggested method (SPE-fluorimetry) | 0.12 ng ml−1 | This work |
| Potentiometry | 77 ng ml−1 | 37 |
| Spectrophotometry | 0.5–28 μg ml−1 | 38 |
| Voltammetry | 4.1 μg ml−1 | 39 |
| Liquid chromatography-mass spectrometric | 1.0 ng ml−1 | 12 |
| High-performance thin-layer chromatography | 1.190 μg ml−1 | 40 |
| Reversed phase HPLC | 0.114 mg l−1 | 10 |
| Liquid chromatography-fluorescence spectroscopy | 2.0 ng ml−1 | 41 |
| Liquid chromatography-tandem mass spectrometry | 1 ng ml−1 | 13 |
| Fluorescence spectroscopy | 0.5 μg ml−1 | 42 |
| HPLC | 20 ng ml−1 | 43 |
4. Conclusion
According to the results, the combination of magnetic solid phase extraction (MSPE), and fluorometry techniques can be considered as an efficient and sensitive approach for the determination of losartan (LOS) in the presence of hydrochlorothiazide (HCTZ) in human urine samples, in part due to the rapidity, sensitivity, low cost, and simplicity of the procedures. The influence of common interfering species in human urine samples such as uric acid, oxalate, phosphate, L-leucine, and glucose was investigated. It is found that these species do not significantly interfere with the proposed method. In addition, the presence of HCTZ has no considerable effect on the determination of LOS via fluorimetric techniques, in part due to its lack of exhibition of fluorescence emission. The limit of detection (LOD) for LOS using this technique was found to be 0.12 ng ml−1 after optimization of the MSPE process.Acknowledgements
We express our gratitude to the Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences for supporting this project.References
- F. Persson, J. B. Lewis, E. Lewis, P. Rossing, N. K. Hollenberg and H. H. Clin, J. Am. Soc. Nephrol., 2011, 6, 1025–1031 CrossRef CAS PubMed.
- D. H. G. Smith, R. Dubiel and M. Jones, Am. J. Cardiovasc. Drugs, 2005, 5, 41–50 CrossRef CAS PubMed.
- R. L. Byyny, J. Hypertens., 1995, 13, S29–S33 CrossRef CAS PubMed.
- P. C. Wong, W. A. Price Jr, A. T. Chiu, J. V. Duncia, D. J. Carini, R. R. Wexler and A. L. Johnson, Br. J. Pharmacol., 1990, 255, 211–217 CAS.
- C. S. Sweet, D. C. Bradstreet, R. S. Berman, N. Jallard, A. Saenz and D. J. Weidler, J. Hypertens., 1994, 7, 1035–1040 CAS.
- P. Sarafidis, P. Georgianos and G. L. Bakris, Nat. Rev. Nephrol., 2013, 9, 51–58 CrossRef PubMed.
- A. Chobanian, N. Engl. J. Med., 2009, 361, 878–887 CrossRef CAS PubMed.
- D. L. Hertzog, J. Finnegan, M. Cafferty, X. Fang, R. J. Tyrrell and R. Ree, J. Pharm. Biomed. Anal., 2002, 30, 747–760 CrossRef CAS PubMed.
- M. Smajic, Z. Vujic, N. Mulavdic and J. Brboric, Chromatographia, 2013, 76, 419–425 CAS.
- R. Bonfilio, C. R. T. Tarley, G. R. Pereira, H. R. N. Salgado and M. B. Araujo, Talanta, 2009, 80, 236–241 CrossRef CAS PubMed.
- A. B. Thomas, U. B. Chavan, R. K. Nanda, L. P. Kothapalli, S. N. Jagdale, S. B. Dighe and A. D. Deshpande, Acta Chromatogr., 2010, 22, 219–226 CrossRef CAS.
- R. N. Rao, S. S. Raju, R. M. Vali and G. G. Sankar, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 902, 47–54 CrossRef CAS PubMed.
- M. Polinko, K. Riffel, H. Song and M. W. Lo, J. Pharm. Biomed. Anal., 2003, 33, 73–84 CrossRef CAS PubMed.
- A. Kumari, H. Prabhakar and R. Giridhar, J. Pharm. Biomed. Anal., 2002, 27, 861–866 CrossRef.
- S. S. El-Hay, M. Y. El-Mammli and A. A. Shalaby, Arabian J. Chem., DOI:10.1016/j.arabjc.2011.06.021.
- I. A. Darwish, Anal. Chim. Acta, 2005, 549, 212–220 CrossRef CAS.
- S. B. Etcheverry, E. G. Ferrer, L. Naso, D. A. Barrio, L. Lezama, T. Rojoc and P. M. Williams, Bioorg. Med. Chem., 2007, 15, 6418–6424 CrossRef CAS PubMed.
- M. R. Balesteros, A. F. Faria and M. A. Oliveira, J. Braz. Chem. Soc., 2007, 18, 554–558 CrossRef CAS.
- Z. Zhang, L. Wang, X. Liu, D. Zhang, L. Zhang and Q. Li, RSC Adv., 2015, 5, 86445–86452 RSC.
- R. Lindberg, G. Fedorova, K. M. Blum, J. P. Prociak, A. Gillman, J. Jarhult, P. Appelblad and H. Soderstrom, Talanta, 2015, 141, 164–169 CrossRef CAS PubMed.
- A. A. Asgharinezhad, N. Mollazadeh, H. Ebrahimzadeh, F. Mirbabaei and N. Shekari, J. Chromatogr. A, 2014, 1338, 1–8 CrossRef CAS PubMed.
- M. E. Mahmoud, S. B. Ahmed, M. M. Osman and T. M. Abdel-Fattah, J. Fuels, 2015, 139, 614–621 CrossRef CAS.
- S. Hahn, J. Traeger and H. J. Holdt, Sep. Sci. Technol., 2015, 50, 191–206 CrossRef CAS.
- K. Boddi, A. Taktsy, S. Szabo, L. Mark, L. Mrko, I. Wittmann, R. Ohmacht, G. Montsko, R. M. Vallant, T. Ringer, R. Bakry, C. W. Huck, G. K. Bonn and Z. Szabo, J. Sep. Sci., 2009, 32, 295–308 CrossRef CAS PubMed.
- M. Jaymand, M. Hatamzadeh and Y. Omidi, Prog. Polym. Sci., 2015, 47, 26–69 CrossRef CAS.
- M. Jaymand, Prog. Polym. Sci., 2013, 38, 1287–1306 CrossRef CAS.
- Z. Huang and H. K. Lee, J. Chromatogr. A, 2015, 1414, 41–50 CrossRef CAS PubMed.
- K. T. Johansen, S. G. Wubshet and N. T. Nyberg, Anal. Chem., 2013, 85, 3183–3189 CrossRef CAS PubMed.
- D. Xiao, C. Zhang, D. Yuan, J. He, J. Wu, K. Zhang, R. Lin and H. He, RSC Adv., 2014, 4, 64843–64854 RSC.
- M. Hatamzadeh, M. Johari-Ahar and M. Jaymand, International Journal of Nanoscience and Nanotechnology, 2012, 8, 51–60 Search PubMed.
- C. Huang and B. Hu, Spectrochim. Acta, Part B, 2008, 63, 437–444 CrossRef.
- A. Dabrowski, Adv. Colloid Interface Sci., 2001, 93, 135–224 CrossRef CAS PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- G. Mckay, H. S. Blair and J. R. Gardener, J. Appl. Polym. Sci., 1982, 27, 3043–3057 CrossRef CAS.
- H. Bagheri, M. Ahmadi, T. Madrakian and A. Afkhami, Sens. Actuators, B, 2015, 221, 681–687 CrossRef CAS.
- S. Vellaichamy and K. Palanivelu, J. Hazard. Mater., 2011, 185, 1131–1139 CrossRef CAS PubMed.
- H. Bagheri, A. Shirzadmehr and M. Rezaei, J. Mol. Liq., 2015, 212, 96–102 CrossRef CAS.
- L. Abdel-Fattah, L. Abdel-Aziz and M. Gaied, Spectrochim. Acta, Part A, 2015, 136, 178–184 CrossRef CAS PubMed.
- M. C. G. Santos, C. R. T. Tarley, L. H. Dall’Antonia and E. R. Sartori, Sens. Actuators, B, 2013, 188, 263–270 CrossRef CAS.
- M. Selvadurai and S. N. Meyyanathan, J. Pharmacol. Toxicol. Methods, 2011, 2, 95–98 Search PubMed.
- M. R. Brunetto, Y. Contreras, S. Clavijo, D. Torres, Y. Delgado, F. Ovalles, C. Ayala, M. Gallignani, J. M. Estela and V. C. Martin, J. Pharm. Biomed. Anal., 2009, 50, 194–199 CrossRef CAS PubMed.
- E. Cagigal, L. Gonzalez, R. M. Alonso and R. M. Jimenez, Talanta, 2001, 54, 1121–1133 CrossRef CAS PubMed.
- C. I. Furtek and M. W. Lo, J. Chromatogr., 1992, 573, 295–301 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |