A comprehensive comparison of bacterial and fungal aerobic granules: formation, properties, surface modification, and biosorption of metals†
Abstract
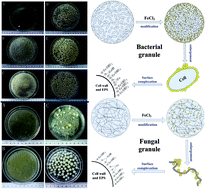
Aerobic granules, a relatively new form of microbial aggregate, can be formed with bacteria or fungi as the dominant population, depending on operational conditions. In this study, a comprehensive comparison is conducted between these two kinds of granules. Bacterial granules (BG) were formed after 3 weeks of cultivation, and exhibited a settling velocity and ash content of 5.6 cm s−1 and 24%, respectively. In contrast, fungal granule (FG) cultivation took only 5 days, and their settling velocity and ash content were 45% and 22% of BG, respectively. BG and FG selectively enriched calcium and potassium, respectively, and these elements were replaced by iron upon Fe(III) modification. Original BG had 7 times larger surface areas and 9 times higher pore volumes than FG, but Fe(III) modification reversed the trend. Original and modified granules were used as biosorbents for removal of Zn(II), Cu(II), Ni(II) and Sb(OH)6− at various pH levels. The original granules removed the cations efficiently (BG better than FG), but had no affinity for Sb(V) under all pH values. Fe(III) modification significantly enhanced Sb(V) removal by both granules, with the best performance achieved under pH 3.4. FG, though it had lower Fe loading, exhibited a comparable ultimate Sb(V) adsorption capacity to BG (111 vs. 125 mg g−1), and 3 times higher initial adsorption rate. In addition, FG was more stable at higher pH and showed better adsorption performance under pH > 4.3. Pseudo-second-order kinetics and a Langmuir isotherm model described the adsorption processes well for both kinds of modified granules, which proved to be good biosorbents.

 Please wait while we load your content...
Please wait while we load your content...