Facile fabrication of multilayer films of graphene oxide/copper phthalocyanine with high dielectric properties†
Abstract
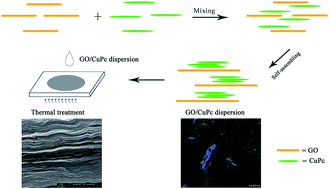
Novel multilayer films of graphene oxide/copper phthalocyanine (GO/CuPc) were fabricated by self-assembling in the orientational ordered liquid crystalline state and immobilizing of the ordered structure upon casting and drying. The formation of the compact and highly ordered structures effectively enhances the dielectric constant of GO/CuPc nanocomposites.

 Please wait while we load your content...
Please wait while we load your content...