Solvent assisted and solvent free orientation of growth of nanoscaled lanthanide sulfides: tuning of morphology and manifestation of photocatalytic behavior†
Abstract
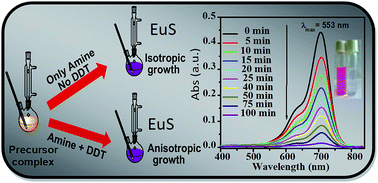
A new class of precursor complexes Ln(acda)3(phen), (where Ln = Nd, Sm, Eu, Tb and Yb, acda− is the anion of 2-aminocyclopentene-1-dithiocarboxylic acid and phen stands for 1,10-phenanthroline) have been used to obtain phase pure lanthanide sulfide nanoparticles by solution-based as well as solution-free thermal treatments at inert conditions. During solution-phase thermolysis, long chain alkyl-amine solvents have been used to promote the reaction at much lower temperature (280 °C) than the solid-phase reaction (650 °C). A contrasting growth feature is observed for nano sulfides and consequently the shape of the material is varied from isotropic cube-like morphology to anisotropic short nanofibers according to the variation of surfactants. The study confirmed that the introduction of 1-dodecanethiol as a structure-modifying capping agent along with reacting amine significantly facilitates the anisotropic growth in a preferred direction. These have been characterized by XRD, TEM, FESEM, UV-vis spectroscopy and BET surface area measurements. The optical absorption data indicated a narrow band gap energy ranging from 1.71–1.97 eV for different EuS. The material emerged as a highly active visible light-driven photocatalyst among the lanthanide sulfides towards the degradation of organic dyes. A comparative catalytic study with morphologically different EuS revealed that the degradation rate changes with varying morphology and for all the dyes it strictly follows the decreasing order of sphere-like particles > cube-like particles > nanofibers.


 Please wait while we load your content...
Please wait while we load your content...