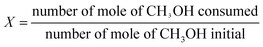Effect of precursor on the performance of phosphate-modified γ-Al2O3 catalysts for the dehydration of methanol
Longkun Xu,
Dan He,
Laishuan Liu*,
Tianjie Pei and
Jun Ren
School of Chemical Engineering and Environment, North University of China, Taiyuan 030051, China. E-mail: liulaishuan@nuc.edu.cn; Fax: +86 351 3922118; Tel: +86 351 3922271
First published on 21st October 2015
Abstract
A series of PO43−/γ-Al2O3 monolith catalysts were prepared by incipient wetness impregnation of phosphate with different additives, including ammonia, triethanolamine (TEA), hexamethylenetetramine (HMT) and diethylenetriamine (MSDS). These catalysts were compared for their catalytic properties in a fixed-bed reactor at 1 atm, 250–350 °C and liquid hourly space velocity (LHSV) of 4 h−1. The catalysts were characterized by nitrogen adsorption, X-ray diffraction (XRD) and temperature-programmed desorption of ammonia (NH3-TPD). The results revealed that the catalytic activity of PO43−/γ-Al2O3 for methanol dehydration increased significantly as the content of phosphate increased to 7 wt%. However, when the phosphate content of PO43−/γ-Al2O3 was further increased to 10 wt%, the activity for methanol dehydration decreased. The catalytic activity of PO43−/γ-Al2O3 is found to be determined mainly by the amounts of weak and medium acid sites. At 7 wt% PO43− loading, the proportion of weak and medium acid sites can be improved by addition of additives with high basicity to the impregnation process. The catalyst containing 7 wt% phosphate with added diethylenetriamine demonstrated the highest activity.
1 Introduction
In addition to being an important intermediate for production of many valuable chemicals such as olefins, methyl acetate and dimethyl sulfate, dimethyl ether (DME) has received global attention since it has great potential as a clean alternative fuel for diesel engines because its thermal efficiency is equivalent to traditional diesel fuel, it has much lower NOx and SOx emissions, near-zero smoke production and so on.1,2 The production of DME via methanol dehydration is more favorable with regards to thermodynamics and economy.3DME is obtained by methanol dehydration reactions using acid catalysts such as zeolite materials, γ-Al2O3 and acid-modified γ-Al2O3. Zeolites are characterized by their high acidity, however, such high acidity can result in significant coke formation and consequently fast deactivation.4–8 Furthermore methanol can undergo secondary reactions to produce hydrocarbons at temperatures higher than 240 °C.9 γ-Al2O3 has been mostly employed due to its low price, easy availability and high stability8 but low activity at temperatures lower than 300 °C.9 Hence, the modification of γ-Al2O3 to increase its catalytic activity for methanol dehydration has become a very important step.
It has been reported that a 10 wt% Nb2O5-modified γ-Al2O3 catalyst exhibits a higher activity for methanol dehydration than the untreated γ-Al2O3 at 240–260 °C.8 Moreover, the sulfate ions incorporated into 10 wt% Nb2O5/γ-Al2O3 increased the amount of acid sites from 1.35 mmol g−1 to 1.68 mmol g−1, which resulted in higher catalytic activity at 240 °C. But the stability of sulfate catalysts was not reported. Khaleel10 found that Ti-modified alumina also showed higher catalytic activity and selectivity for methanol to DME at temperatures ≤220 °C. Yaripour and Baghaei11 reported that the silica modified γ-Al2O3 catalysts also showed better performance for the methanol dehydration reaction, in which the sample with 6 wt% silica loading exhibited the best methanol conversion.
Mao1 found that 10 wt% SO42−/γ-Al2O3 calcined at 550 °C exhibited the high selectivity and yield for DME from syngas. Yaripour and Baghaei11 concluded that phosphorus-modified catalysts have shown better performance compared to the untreated γ-Al2O3 for methanol to DME. Panpranot12 reported that amorphous AlPO4 catalyst pretreated at 200–300 °C with steam exhibited higher methanol conversion than non-treated catalyst.
Concerning the acid strength, some authors13,14 suggested (mostly based on NH3-TPD profiles) that acid sites of medium strength are the most desirable from the viewpoint of DME selectivity, while strong acid sites do promote the formation of by-products at the expense of DME; others,11 however, stated that acid sites of relatively weak and medium strength (again based on NH3-TPD results) can also efficiently convert methanol to DME or even that weak acid sites are more effective for the selective DME synthesis.2
It is apparent from the above discussions that a systematic work is required in order to gain more insights into the influence of the acid property of the dehydration catalysts. For this purpose, we have prepared a series of PO43−/γ-Al2O3 catalysts in which the γ-Al2O3 acidity was systematically varied by different amount and types of additives. The acid properties of the samples have been measured by NH3-TPD. The effects of phosphorus on the catalytic activity have been investigated. In addition, the activity was correlated with the amount of surface acid sites of these catalysts.
2 Experimental
2.1 Preparation of catalysts
The second series of samples was prepared following the above procedure, but (NH4)2HPO4 was substituted with 28% ammonium hydroxide and phosphoric acid. This series of samples was denoted A(y)-7PO43−/Al2O3, where A and y stand for the ammonium hydroxide and n(NH3)/n(PO43−) ratios of impregnation solution, respectively.
The third type of catalysts was also prepared following the above procedure, but ammonium hydroxide was substituted with TEA, HMT and MSDS. This series of samples with TEA, HMT and MSDS were named T(3)-7PO43−/Al2O3, H(3)-7PO43−/Al2O3 and M(3)-7PO43−/Al2O3, respectively. 3 means the mole ratios of nitrogen atoms in additives to H3PO4.
2.2 Characterization of the supports and catalysts
Pore characteristics of the samples were obtained from adsorption–desorption isotherms of nitrogen at 77 K with a Micromeritics ASAP2000 apparatus. The specific surface areas were calculated using BET equation. The total pore volume was calculated from a single point on the nitrogen adsorption isotherm at the relative pressure of about 0.995.15The XRD patterns of the prepared catalysts were measured from10° to 80° 2θ using an X-ray diffract meter (Rigaku Dmax-2000) with Cu-Kα radiation. Prior to the XRD analyses, the samples were grinded to sizes smaller than 0.1 mm.
The NH3-TPD was performed in a Micromeritic Chemisorb2020 equipped with a thermal conductivity detector (TCD). Before adsorption, the sample was pretreated in high purity N2 (30 cm3 min−1) at 550 °C for 1 h. Then, it was saturated with 5% NH3/He at 100 °C for 1 h (30 cm3 min−1) and subsequently flushed with flowing He (30 cm3 min−1) at 100 °C for 1 h to remove physisorbed NH3. The NH3-TPD was carried out from 100 to 600 °C at a constant heating rate of 5 °C min−1.
2.3 Catalytic tests
The vapor phase dehydration reaction was carried out in a flow fixed-bed reactor (stainless-steel tube i.d., 10 mm; length, 240 mm). In an experiment, 4.0 g of catalyst (size, 40–60 mesh; volume, 9 ml) was loaded in the middle section of the reactor tube. Then, methanol was pumped with LHSV of 4 h−1 by a set of metering pumps (ILSHIN Autoclave Co., Ltd.), preheated to temperature of 240 °C and introduced into the reactor. The reaction products were analyzed on line with a gas chromatograph (HP 4890D) equipped with a compacted column (Carboxen 1000, 3 m × 0.3 cm) connected to a TCD detector, where inorganic gases (CO and CO2) were measured; and with a compacted column (Porapak-Q, 2.5 m × 0.3 cm) connected to a flame ionization detector to analyze organic compound (CH3OH, CH3OCH3, light hydrocarbon).16 Methanol conversion (X) and product selectivity (S) were calculated as follows:Methanol conversion:
DME selectivity:
3 Results and discussion
3.1 Characterizations of the samples
Table 1 summarizes the specific BET surface areas, pore volumes and pore diameters of the catalysts employed in this paper. The specific surface area and pore volume of γ-Al2O3 support are 325 m2 g−1 and 0.58 cm3 g−1, respectively. The decrease of surface areas and pore volumes with the increase of PO43− content may be caused by the formation of AlPO4.| Sample | SA (m2 g−1) | VP (cm3 g−1) | dP (nm) | Aciditya (mmol g−1) | |||
|---|---|---|---|---|---|---|---|
| Weakb | Moderatec | Strong d | Total | ||||
| a Determined by NH3-TPD.b Fitted peak located at 230 ± 20 °C in Fig. 2.c Fitted peak located at 290 ± 20 °C in Fig. 2.d Fitted peak located at 400 ± 30 °C in Fig. 2. | |||||||
| γ-Al2O3 | 325 | 0.58 | 7.1 | 0.15 | 0.22 | 0.31 | 0.68 |
| 3PO43−/Al2O3 | 317 | 0.55 | 6.9 | 0.16 | 0.28 | 0.40 | 0.84 |
| 7PO43−/Al2O3 | 307 | 0.50 | 6.5 | 0.18 | 0.32 | 0.60 | 1.10 |
| 10PO43−/Al2O3 | 264 | 0.40 | 6.1 | 0.18 | 0.34 | 0.62 | 1.14 |
| A(1)-7PO43−/Al2O3 | 305 | 0.49 | 6.4 | 0.17 | 0.30 | 0.61 | 1.08 |
| A(2)-7PO43−/Al2O3 | 307 | 0.50 | 6.5 | 0.19 | 0.34 | 0.59 | 1.12 |
| A(3)-7PO43−/Al2O3 | 308 | 0.51 | 6.6 | 0.21 | 0.38 | 0.54 | 1.13 |
| T(3)-7PO43−/Al2O3 | 304 | 0.48 | 6.3 | 0.18 | 0.31 | 0.60 | 1.09 |
| H(3)-7PO43−/Al2O3 | 306 | 0.50 | 6.5 | 0.20 | 0.38 | 0.52 | 1.10 |
| M(3)-7PO43−/Al2O3 | 310 | 0.52 | 6.7 | 0.31 | 0.38 | 0.48 | 1.17 |
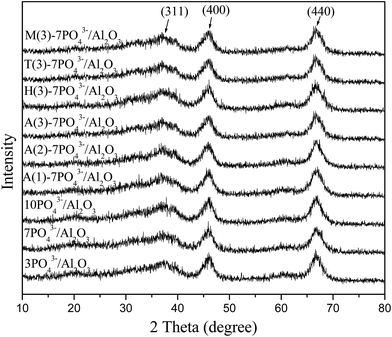
The XRD patterns of the phosphorus-modified γ-Al2O3 samples are shown in Fig. 1. We can see that all the samples have a crystallite γ-Al2O3 phase (JCPDS no: 1-1307). These results indicated that the AlPO4 in amorphous form highly dispersed on the surface of γ-Al2O3.
The surface acidic properties of the catalysts were characterized by NH3-TPD, as shown in Fig. 2. In all cases, NH3-TPD patterns are characterized by an intense desorption peak located at ca. 200 °C and abroad asymmetric decaying “tail” which extends up to ca. 600 °C. Qualitatively similar NH3-TPD profiles are typically observed for different crystalline phases of γ-Al2O3.12,17,18 In order to determine the acid strength and the amount of acid sites on catalyst surface, the NH3-TPD profiles were deconvoluted into Gaussian peaks, on the assumptions that (a) the activation energy of desorption (Ed) is constant and independent of surface coverage, and (b) a normal distribution of Ed arises from a corresponding heterogeneity of sites distribution.12,19 The fitting procedure showed that all experimental curves may be de-convoluted into three peaks, which located at 230 ± 20 °C (peak I), 290 ± 20 °C (peak II) and 400 ± 30 °C (peak III), respectively. Peaks I and II may be attributed to desorption of NH3 from weak and medium acid sites, whereas peak III corresponds to strong acid sites.17,20–22 The number of acid sites in these samples was calculated and is summarized in Table 1.
For the xPO43−/Al2O3 samples, the number of the total acid sites was increased with phosphate content. Excessive phosphate loading cause the blockage of pores and significantly increase the strong acid sites (Table 1).
For the A(y)-7PO43−/Al2O3 samples, desorption peak was shifted to low desorption temperatures, suggesting that the acid strength of the samples decreased with the increase of the n(NH3)/n(PO43−) ratios. Moreover, the number of weak and medium acid sites increase with the n(NH3)/n(PO43−) ratios.
It is quite interesting to note that the amounts of weak and medium acid sites decreased as follows: M(3)-7PO43−/Al2O3 > A(3)-7PO43−/Al2O3 > H(3)-7PO43−/Al2O3 > T(3)-7PO43−/Al2O3. The sequence accords with the alkalinity of the additives (MSDS, pKa = 10.10; ammonia, pKa = 9.24; TEA, pKa = 7.82 and HMT, pKa = 5.15). This result suggests that the additive with high basicity would be in favour of the formation of highly dispersed AlPO4.
3.2 Catalytic activity
The activity of catalysts as functions of reaction temperatures is shown in Fig. 3. For comparison, the equilibrium conversion curve for methanol dehydration to DME is also shown in Fig. 3.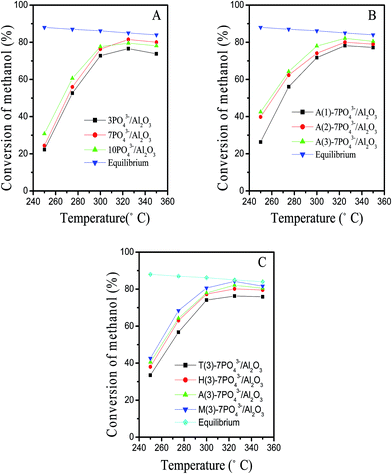 | ||
| Fig. 3 Catalytic test of catalyst with variation of (A) phosphate content (B) n(NH3)/n(PO43−) ratios (C) additives. | ||
As shown in Fig. 3A, the conversion of methanol increases with the increase of the PO43− content at temperature below 300 °C. The acceleration of the catalytic performance was attributed to an increase of the acid sites.1 Moreover, the 3PO43−/Al2O3 and 7PO43−/Al2O3 catalysts are 100% selective to DME within the range of temperatures studied here. Above 325 °C, the methanol conversion on 10PO43−/Al2O3 catalyst is lower than that of the other phosphate-modified γ-Al2O3 catalysts and the selectivity to DME for 10PO43−/Al2O3 decrease from 100% to 96%. This phenomenon can be attributed to blocking the active acid sites of γ-Al2O3 with phosphorus (Table 1). On the other hand, the high amount of strong acid sites do promote the formation of by-products at the expense of DME.4,11
In the temperature region of 275 to 350 °C, the activity of catalysts with different n(NH3)/n(PO43−) ratios and additive is improved in order of A(3)-7PO43−/Al2O3 > A(2)-7PO43−/Al2O3 > A(1)-7PO43−/Al2O3 and M(3)-7PO43−/Al2O3 > A(3)-7PO43−/Al2O3 > H(3)-7PO43−/Al2O3 > T(3)-7PO43−/Al2O3, respectively, which is in good agreement with the increasing order of the number of weak and medium acidic sites. Their selectivity of DME (not shown) is nearly 100%. This indicates that the number of weak and medium acidic sites plays an important role on the catalytic activity for methanol dehydration.11
In order to verify above results, Fig. 4 depicts the methanol conversion as a function of the amount of weak and medium acid sites. It is clearly showing a linear relationship between these two parameters in reaction temperature region of 250–325 °C. This is in agreement with results of previous studies,11 which reported the strength of weak and moderate acid sites is a crucial factor for catalytic activity of methanol dehydration.
3.3 Catalyst stability test
The stability of A(3)-7PO43−/Al2O3 and M(3)-7PO43−/Al2O3 catalyst were measured over a 50 h period. The operating temperature was 325 °C and the LHSV was 4 h−1. Fig. 5 clearly showed that the methanol conversion remained constant for the whole period, which indicated that no noticeable deactivation of the two catalysts was occurring. This result revealed that the phosphate modified γ-Al2O3 had excellent stability for the synthesis of DME from methanol.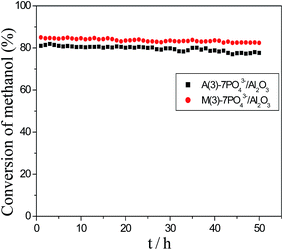 | ||
| Fig. 5 Activity and stability of the PO43−/Al2O3 catalysts at 235 °C. (1) A(3)-7PO43−/Al2O3; (2) M(3)-7PO43−/Al2O3 LHSV = 4 h−1. | ||
4 Conclusions
A series of solid acid catalysts were prepared by impregnation method. The phosphate-modified γ-Al2O3 catalysts were used for the methanol dehydration reaction. The conversion of methanol was enhanced by PO43− modification and the number of acid sites was increased by the modification. Furthermore, the proportion of weak and medium acid sites can be improved by high alkaline additives. The M(3)-7PO43−/Al2O3 catalyst exhibited the highest reactivity, selectivity and stability for the synthesis of DME from methanol. The high activity, selectivity and long time stability can be attributed to the high amount of weak and medium acid sites.Acknowledgements
The authors are thankful for the financial support by Shanxi Province science and technology major projects (20111101013), the natural science foundation of Shanxi Province project (2009011011-4).References
- D. S. Mao, W. M. Yang, J. C. Xia, B. Zhang and G. Z. Lu, J. Mol. Catal. A: Chem., 2006, 250, 138–144 CrossRef CAS.
- L. Liu, W. Huang, Z. H. Gao and L. H. Yin, J. Ind. Eng. Chem., 2012, 18, 123–127 CrossRef CAS.
- S. M. K. Aboul-Fotouh, J. Fuel Chem. Technol., 2013, 41(9), 1077–1084 CrossRef.
- L. W. Zhang, J. H. Wang, P. Wu, Z. Y. Hou, J. H. Fei and X. M. Zheng, Chin. J. Catal., 2010, 31, 987–992 CrossRef CAS.
- N. Khandan, M. Kazemeini and M. Aghaziarati, Appl. Catal., A, 2008, 349, 6–12 CrossRef CAS.
- Y. Tan, H. Xie, H. Cui, Y. Han and B. Zhong, Catal. Today, 2005, 104, 25–29 CrossRef CAS.
- K. C. Tokay, T. Dogu and G. Dogu, Chem. Eng. J., 2012, 184, 278–285 CrossRef CAS.
- D. H. Liu, C. F. Yao, J. Q. Zhang, D. Y. Fang and D. S. Chen, Fuel, 2011, 90, 1738–1742 CrossRef CAS.
- Y. Fu, T. Hong, J. Chen, A. Auroux and J. Shen, Thermochim. Acta, 2005, 434, 22–26 CrossRef CAS.
- A. Khaleel, Fuel, 2011, 90, 2422–2427 CrossRef CAS.
- F. Yaripour, F. Baghaei and I. Schmidt, Catal. Commun., 2006, 6, 542–549 CrossRef.
- K. Lertjiamratn, P. Praserthdam, M. Arai and J. Panpranot, Appl. Catal., A, 2010, 378, 119–123 CrossRef CAS.
- A. R. Keshavarz, M. Rezaei and F. Yaripour, J. Nat. Gas Chem., 2011, 20, 334–338 CrossRef CAS.
- Z. Hosseini, M. Taghizadeh and F. Yaripour, J. Nat. Gas Chem., 2011, 20, 128–134 CrossRef CAS.
- L. S. Liu, Z. Y. Liu, J. L. Yang, Z. G. Huang and Z. H. Liu, Carbon, 2007, 45, 2836–2842 CrossRef CAS.
- R. M. Ladera, J. L. G. Fierro, M. Ojeda and S. Rojas, J. Catal., 2014, 312, 195–203 CrossRef CAS.
- S. S. Akarmazyana and P. Panagiotopouloua, Appl. Catal., B, 2014, 145, 136–148 CrossRef.
- J. Khom-in, P. Praserthdam, J. Panpranot and O. Mekasuwandumrong, Catal. Commun., 2008, 9, 1955–1958 CrossRef CAS.
- F. Arena, R. Dario and A. Parmaliana, Appl. Catal., A, 1998, 170, 127–137 CrossRef CAS.
- S. M. Kim, Y.-J. Lee, J. W. Bae, H. S. Potdar and K.-W. Jun, Appl. Catal., A, 2008, 348, 113–120 CrossRef CAS.
- E. Kraleva, R. Palcheva, L. Dimitrov, U. Armbruster, A. Brückner and A. Spojakina, J. Mater. Sci., 2011, 46, 7160–7168 CrossRef CAS.
- G. R. Moradi, F. Yaripour and P. Vale-Sheyda, Fuel Process. Technol., 2010, 91, 461–468 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |