Single-process fabrication of antireflective acrylic hard coating via surface segregation of porous silica nanoparticles†
Tomoyo Shimogakiab,
Hiroki Tokorob,
Minoru Tabuchib,
Naoto Inoueb,
Takuji Tsukamotob,
Toru Ishiib,
Nobuyuki Koikeb,
Yohzoh Yamashinab and
Masahide Takahashi*a
aDepartment of Materials Science, Graduate School of Engineering, Osaka Prefecture University, Sakai, Osaka 599-8531, Japan. E-mail: masa@photomater.com
bDIC Corporation, Ichihara, Chiba 290-8585, Japan
First published on 7th December 2015
Abstract
A new type of antireflective (AR) acrylic hard coating consisting of a surface-segregated single layer of silica nanoparticles and acrylic polymer matrix is proposed that exhibits good mechanical stability and AR capability. In addition, the polymer hard coating can be obtained by a single coating process. Porous silica particles 100 nm in diameter with surfaces modified by hexamethyl disilazane (HMDS) undergo spontaneous segregation at the air interface of the acrylic monomer film. The monolayer of porous silica particles can serve as an AR surface; a reflectance of 2.7% at 550 nm and a pencil hardness > H are achieved. The great advantage of the present approach is that a hard coating with AR capability can be obtained from an acrylic monomer solution containing porous silica nanoparticles via a presently available general polymer coating process. The present approach to an AR hard coating offers a much easier and simpler way than the conventional ones, which require multi-step coating or nanolithography.
Introduction
Antireflective (AR) coatings are an important class of technology used to improve the quality of our daily life by offering a better visibility for flat panel displays, mobile phones, and tablets by reducing the reflection of surrounding light.1–3 Two types of AR coating have been reported and industrialized so far. The optical reflection is suppressed by surface microstructures or coatings with a refractive index between the window materials and air, which decreases the refractive index contrast at the air interface.4–20 Another type of AR coating is designed to cancel the optical reflection by optical interference within the multilayers of alternating refractive indices.21–23 A typical example of the former AR surface is the moth's eye structure.10–16,24 This structure mimics the eyes of moths, in which micro cones ∼100 nm in diameter and several hundreds of nm in height with 100 to 200 nm regular spacings are fabricated on the entire surface of the display devices. The refractive index of the moth's eye surfaces gradually changes from that of air to that of the window material. The optical reflection is suppressed because of the absence of a refractive index jump at the interfaces. The moth's eye structure shows an excellent AR capability. However, the sophisticated lithographic technique at the 100-nm scale is required to fabricate the moth's eye structure. In addition, such micro structures are very fragile and do not survive mechanical contact by human fingers or other objects. Furthermore, the substrate is limited to hard materials such as glass because they need to be capable of nanolithography. Further improvement in mechanical robustness is strongly required for moth's eye-structured AR surfaces. The multilayer optical thin film is widely used for displays or glasses presently available because of their simple structure and better surface mechanical properties.21–23 The use of the porous silica nano particles as AR coating is also reported by several literatures.18,25 By preparing an optical thin film with a designated refractive index and thickness, the two reflections at the air/film and film/device interfaces interfere each other, resulting in the cancelation of the reflection of light through an anti-phase interaction. Low-refractive index coatings are usually required for this type of AR coating. However, the low refractive index layer is required to be coated at a thickness of 100 nm with an accuracy of several nanometers, which is technologically very difficult from an industrial point of view. In addition, the properties of this type of AR coating exhibit a strong dependence on the incidence angle. In addition, the size of displays are increasing day by day, but their thickness and weight are decreasing, so the surface materials of displays are replacing from glass to hard coat resins. Roll-to-roll process is practically used for such large-sized coating. Therefore, an easier and simpler method for robust resin AR coatings for large area exceeding several tens to hundreds inches is strongly required from both industrial and practical points of view.In the present study, a hard coating acrylic polymer film with a surface-segregated porous silica nanoparticle layer by a single bar-coating process which can be easily transformed into a roll-to-roll fabrication has been proposed as a new type of AR coating possessing mechanical robustness coupled with excellent optical quality. The surface porous silica layer embedded in the resin serves as a surface low-refractive index layer that does not deteriorate and demonstrates the mechanical robustness of a polymer hard coating. One of the advantages of the present approach is that the substrate materials are not limited, which means they can be used for variety of applications. In addition, such composite films can be formed via a single coating process. The surface porous silica particle layer is formed through spontaneous self-organization during the coating process. When the surface free energy of silica particles is lower than that of acrylic monomers, which can be considered a polymer hard coating, the particles will eventually segregate on the polymer/air interface to minimize the interfacial energy. The surface tension of acrylic monomers is in the range of 30 to 50 mN m−1.26 By optimizing the surface chemistry of nano silica particles, single-layered homogeneous segregation can be formed at the polymer/air interface. A conceptual schematic of the present approach is depicted in Fig. 1. It is difficult to control such surface segregation with bare silica particles because the surface silanol group has a high polarity and a large surface free energy (>100 mN m−1). Reducing the surface energy of the silica particles is required to induce the segregation at the polymer/air interface. The surface energy of the porous silica nanoparticles can be reduced by grafting a perfluoroalkyl group on the surface. The trifluoromethyl group (–CF3) in perfluoroalkyl shows the lowest critical surface tension (6 mN m−1) among organic functional groups. The particles modified with a –CF3 group are expected to segregate at the air interface to minimize the interfacial energy. Recently, however, the use of fluoro-compounds has been greatly limited because of some concerns for the safety of the human body and environment. Therefore, the use of non-fluorinated surface grafting agents to adjust the surface energy is required. The surface energy of a methyl group has been reported to be 22 to 24 mN m−1,27,28 so that grafting agents with methyl groups on a silica surface decreases the surface energy below that of acrylic monomers.
In the present study, porous silica particles were modified with hexamethyldisilazane (HMDS). To investigate the relationship between the silica coverage factor of HMDS and the tendency of surface segregation, acrylic hard coatings containing modified porous silica nano particles with various surface coverages were prepared. Hollow nano silica particles were also used for comparison. The uniform segregation of porous silica particles at the air interface was carefully monitored to achieve a new type of AR hard coating.
Experimental method
Materials
Methanol (Kanto Chemical Co. Inc., Japan), 2-propanol (IPA; Kanto Chemical Co. Inc., Japan), methyl ethyl ketone (MEK; Kanto Chemical Co. Inc., Japan), tetramethoxysilane (TMOS; Shin-Etsu, Japan), dodecylamine (DDA; TCI, Japan), decylamine (DA; TCI, Japan), 28% aqueous ammonia (NH3, Wako Inc., Japan), acetic acid (TCI, Japan), hexamethyldisylazane (HMDS; TCI, Japan), Nano Balloon XL100 (hollow silica spheres, GRANDEX, Japan), ARONIX M-306 (hard acrylic coating: mixture of pentaerythritol triacrylate and pentaerythritol tetraacrylate; TOAGOSEI Co., LTD., Japan), IRGACURE 184 (photoinitiator, BASF Canada Inc., Canada), and MEGAFACE F-444 (DIC, Japan) were used as received.Synthesis
Experiments shown in Fig. 6 and S-6† were carried out with silica particles prepared with a different chemical composition. They were 100 nm in diameter and had a specific surface energy of 100 m2 g−1. The pore volume was 0.04 cm3 g−1, with an estimated pore volume of ca. 10%. The synthetic details are shown in the ESI.†
Results and discussion
Surface modification
Microporous and monodispersed silica nanoparticles 100 nm in diameter were synthesized following the procedure that we have reported recently.29,30 Commercial hollow silica particles 50 to 60 nm in diameter (Nano Balloon XL100) were used for a comparison. Porous or hollow silica particles are considered to be the most suitable candidates for surface particle layers. However, bare silica particles show a poor affinity with acrylic monomers/resins because of their strong cohesion force and large difference in surface energy. For example, the critical surface tensions of silica particles and general acrylic resins are >100 mN m−1 and 30 to 50 mN m−1, respectively. It is thus necessary to modify the surface of silica particles by grafting molecules with a lower surface energy while maintaining good affinity with the surrounding media. HDMS was selected as the grafting molecules to reduce the surface energy of the particles. If the entire surface of silica particles is covered with –CH3, the critical surface tension becomes 22 to 24 mN m−1.27,28 For hollow silica particles, the particles were broken by a large shear force during the wet jet milling process (Fig. S-1†). Even though hollow silica particles seem to be the best candidate material for the surface layer because of their small refractive index, their decreased mechanical stability makes them unusable for the present purpose. Accordingly, the following experiments were carried out only with the microporous silica particles.By surface modification of microporous silica particles, micro pores might be filled with HDMS. The relationship between the theoretical coverage of HMDS and the pore volume of the modified silica particles is shown in Fig. 2. The theoretical coverage was calculated using eqn (1):
 | (1) |
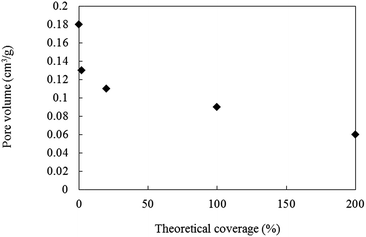 | ||
| Fig. 2 Relationship between theoretical coverage of microporous silica particles modified with various amount of HMDS and measured pore volume. | ||
The theoretical area of the HMDS molecule is assumed to be 967 m2 g−1,31 and the amount of HMDS is experimentally estimated by measuring the consumed quantity of HDMS during the surface modification process. The pore volume of porous silica decreased with the surface coverage, in other words, with increasing amounts of HDMS. It should be noted that the HMDS modifies not only the outermost surface of silica particles but also inside the pores. In the case of particles with 100% coverage of HDMS, the pore volume decreased to half that of bare particles. Practical amount of HDMS grafting on the surface of nano silica is hardly quantified, but thermal gravity analyses indicate the larger the amount of HDMS are grafted for porous silica with higher the theoretical area (Fig. S-2†).
Coating
The IPA-MEK acrylic monomer solution of ARONIX M-306 and IRGACURE containing silica particles modified with various amount of HMDS was coated on PET film substrates by bar-coating to determine the surface segregation tendency. ARONIX M-306 contains pentaerythritol triacrylate and pentaerythritol tetraacrylate monomers (PETA), which is known to be one of the general multifunctional acrylic resins for hard coatings (Fig. S-3†).Cross-sectional TEM and surface SEM images of coating films are shown in Fig. 3 and 4, respectively. AFM images of the surface of coating films and their haze are shown in Fig. S-4.† The non-modified silica particles are dispersed almost homogeneously in the hard coating (Fig. 3(a)). However, the modified particles exhibit single-layer segregation of silica particles at the air interface (Fig. 3(b) and (c)), leaving a certain fraction of the particles on the interior of the films. In TEM images, the amount of surface segregation is almost the same for HDMS coverages of 20% and 100%, where the rates of particles segregation at the air interface were ca. 40% in both cases. In contrast, the rate of bare particles is 4%. A 20% coverage of HMDS on silica particles seems to be sufficient for the formation of the surface segregated layer. With the present approach, the surface layer of silica nanoparticles can be spontaneously formed through the segregation via a surface energy mismatch between the particles and the acrylic monomers. As a result, the air interface is almost completely covered by a single layer of silica nanoparticles.
 | ||
| Fig. 3 Cross sectional TEM images of coating films containing porous silica particle with various surface coverages of HMDS; (a) 0, (b) 20 and (c) 100 area%. | ||
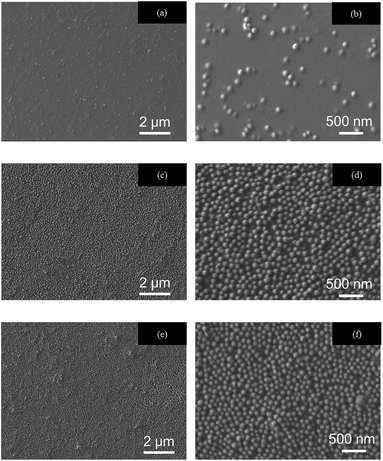 | ||
| Fig. 4 SEM images of the surface of coating films containing porous silica particle with various surface coverages of HMDS; (a, b) 0, (c, d) 20 and (e, f) 100 area%. | ||
The theoretical AR capability of the present system was examined by theoretical simulation (Fig. S-5 and S-6†). Using silica particles with 40% and 50% pore volume, the reflectance was calculated to be less than 1%. In other words, the present coatings show great potential as AR coatings. The AR property of these films was also experimentally examined. Reflectance spectra were measured for samples prepared with silica nanoparticles with various surface coverages of HDMS (Fig. 5). The reflectance at 550 nm, which is a highly visible wavelength, and total light transmission are shown in Table 1.
 | ||
| Fig. 5 The reflectance spectra of coating films containing silica particles modified by HMDS with a coverage of (a) 0, (b) 20, (c) 100 area%. | ||
| Theoretical coverage (%) | 0 | 20 | 100 |
| Reflectance at 550 nm (%) | 4.1 | 3.0 | 2.7 |
| Total light transmission (%) | 90.3 | 91.0 | 91.1 |
All films showed >90% transmission, which is sufficiently transparent for display applications. Furthermore, the pencil hardness of these films were H–3H, even on PET film as a substrate, demonstrating that these films are highly scratch-resistant, similar to the acrylic coating without silica particles. The reflectance of films with a silica particle layer was <3%, lower than that of the film with bare silica particles (4.1%). The reflectance of the 100 area% film is larger than the theoretical prediction. This may be due to the in-plane inhomogeneity of the surface silica layer and reduction of the pore volume by HMDS modification, which could be further improved by optimization.
The reflectance of porous silica with 100 area% HMDS coverage showed a better AR property, 2.7%. However, the rate of segregation was the same as that of 20 area% HMDS coverage. Comparing SEM images of the surface of coating films, the 20% coverage of HMDS on silica particles seems to have a rougher surface (inhomogeneous distribution of silica particles) (Fig. 4(d)) than the one with 100% coverage (Fig. 4(f)). It was also observed that some of the particles seem to be stacked up. This inhomogeneity may influence the reflectance difference between the 20 and 100 area% films. These silica particles can be segregated easily at the air surface, reducing the optical reflection at the surface of the coating film. The same experiments were carried out with silica particles of the same diameter but different porosity to see the effect of the density of the particles (Fig. S-7†). Even with silica particles with larger density (heavier than the previous experiments shown above), surface segregation of silica particles is achieved. Furthermore, only one layer was formed, and the excessive silica particles were dispersed in coating resins instead of forming a second layer (Fig. S-7(b)†).
To confirm the hypothesis that the segregation is due to the differences in the surface free energy, a fluoro-surfactant molecule (MEGAFACE F-554), the surface energy of which is lower than that of HMDS-modified silica particles, was incorporated into the acrylic monomer-silica particle solution (Fig. 6). It is expected that the fluoro-surfactant molecules will tend to accumulate at the air interface because of the large surface energy difference. In the case of 1.0 wt% fluoro-surfactant-containing film, the segregation of silica particles was no longer observed. Even with 0.1 wt% fluoro-surfactant addition, the number of surface silica particles drastically decreased compared with the cases shown in Fig. 3(a) and (b). These observations indicate that, once the entire surface is covered by the single layer of silica particles or another surfactant with lower surface energy than silica particles, the remaining particles are immobilized in the matrix because of elimination of the air interface by the silica or the surfactant layer. This result also indicates that the surface segregation of silica particles is driven by the surface energy difference. The possibility of phase separation and buoyant force can be ruled out. Once the surface single layer of silica nanoparticles formed, the segregation did not proceed further, resulting in the spontaneous formation of the silica particle single layer at the air interface. In addition, even if the excess amount of silica particles remains in the film matrix, they do not alter the optical transparency of the film because they are small compared with the wavelength of visible light.
 | ||
| Fig. 6 TEM images of the cross section of coating films containing porous silica particle modified with HMDS and (a) 0.1 and (b) 1.0 wt% fluoro-surfactant. | ||
To consider the segregation mechanism in more detail, the amount of solvent in the coating liquid was decreased from 60 to 50 and 45 wt%. SEM images of the surfaces of the obtained coating films are shown in Fig. 7.
 | ||
| Fig. 7 SEM images of surfaces of coating film containing porous silica particles modified with HMDS; (a) 45, (b) 50 and (b) 60 wt% solvent ratio. | ||
As the amount of solvent decreased, the number of segregated silica particles decreased. This result indicates that the silica particles were segregated gradually under the convective flow during the dry coating process. Specifically, the silica particles were transferred to the air interface by convective flow. Once the silica arrived at the air interface, it was immobilized because the interface energy difference was unfavourable.
Conclusions
Porous silica particles 100 nm diameter with surfaces modified by HMDS can segregate spontaneously at the air surface to form a monolayer. The obtained hard-coating film shows AR properties. In this film, the monolayer of porous silica particles can form a low-refractive index layer. HMDS was found to be an effective modifier to segregate the particles at the air surface. One of the advantages of the present approach is that fluoro modifier is no longer required to adjust the surface energies. A reflectance of 2.7% and pencil hardness of > H on a PET substrate were achieved. Another advantage is that the silica monolayer can serve as a low-refractive index 100-nm layer. The present approach offers an easier and simpler method for fabricating a robust AR coating for a variety of applications.Acknowledgements
This work was partly supported by a Grant-in-Aid for Scientific Research (B) (26288108), a Grant-in-Aid for Scientific Research on Innovative Area (26630322) from the Ministry of Education, Culture Sports, Science and Technology of Japan.Notes and references
- P. B. Clapham and M. C. Hutley, Nature, 1973, 244, 281 CrossRef
.
- C. G. Bernhard, Endeavour, 1967, 26, 79 Search PubMed
.
- G. S. Lim, H. Kim and J. Y. Chang, Energy Environ. Sci., 2011, 3, 3779 Search PubMed
.
- D. G. Chen, Sol. Energy Mater. Sol. Cells, 2001, 68, 313 CrossRef CAS
.
- W. Joo, H. J. Kim and J. K. Kim, Langmuir, 2010, 26, 5110 CrossRef CAS PubMed
.
- Y. Liu, T. Lai, H. Li, Y. Wang, Z. Mei, H. Liang, Z. Li, F. Zhang, W. Wang, A. Y. Kuznetsov and X. Du, Small, 2012, 8, 1392 CrossRef CAS PubMed
.
- B. Daglar, T. Khudiyev, G. B. Demirel, F. Buyukserin and M. Bayindir, J. Mater. Chem. C, 2013, 1, 7842 RSC
.
- X. Li, J. Gao, L. Xue and Y. Han, Adv. Funct. Mater., 2010, 20, 259 CrossRef CAS
.
- A. Yildirim, T. Khudiyev, B. Daglar, H. Budunoglu, A. K. Okyay and M. Bayindir, ACS Appl. Mater. Interfaces, 2015, 7, 326 Search PubMed
.
- P. B. Clapham and M. C. Hutley, Nature, 1973, 244, 281 CrossRef
.
- J. Nishii, K. Kintaka, Y. Kawamoto, A. Mizutani and H. Kikuta, J. Ceram. Soc. Jpn., 2003, 111, 24 CrossRef CAS
.
- Y. Li, J. Zhang, S. Zhu, H. Dong, F. Jia, Z. Wang, Z. Sun, L. Zhang, Y. Li, H. Li, X. Xu and B. Yang, Adv. Mater., 2009, 21, 4731 CAS
.
- Y. Li, J. Zhang and B. Yang, Nano Today, 2010, 5, 117 CrossRef
.
- S. Chattopadhyay, Y. F. Huang, Y. J. Jen, A. Ganguly, K. H. Chen and L. C. Chen, Mater. Sci. Eng., R, 2010, 69, 1 CrossRef
.
- H. Deniz, T. Khudiyev, F. Buyukserin and M. Bayindir, Appl. Phys. Lett., 2011, 99, 183107 CrossRef
.
- S. Kim, U. T. Jung, S.-K. Kim, J.-H. Lee, H. S. Choi, C.-S. Kim and M. Y. Jeong, ACS Appl. Mater. Interfaces, 2015, 7, 326 CAS
.
- B. G. Prevo, Y. Hwang and O. D. Velev, Chem. Mater., 2005, 17, 3642 CrossRef CAS
.
- Y. Hoshikawa, H. Yabe, A. Nomura, T. Yamaki, A. Shimojima and T. Okubo, Chem. Mater., 2010, 22, 12 CrossRef CAS
.
- H. Budunoglu, A. Yildirim and M. Bayindir, J. Mater. Chem., 2012, 22, 9671 RSC
.
- K. Katagiri, S. Yamazaki, K. Inumaru and K. Koumoto, Polym. J., 2015, 47, 190 CrossRef CAS
.
- J. A. Hiller, J. D. Mendelsohn and M. F. Rubner, Nat. Mater., 2002, 1, 59 CrossRef CAS
.
- S. Kim, J. Cho and K. Char, Langmuir, 2007, 23, 6737 CrossRef CAS
.
- Y. Du, L. E. Luna, W. S. Tan, M. F. Rubner and R. E. Cohen, ACS Nano, 2010, 4, 4308 CrossRef CAS
.
- C. G. Bernhard, Endeavour, 1967, 26, 79 Search PubMed
.
- J. Moghal, J. Kobler, J. Sauer, J. Best, M. Gardener, A. A. R. Watt and G. Wakefield, ACS Appl. Mater. Interfaces, 2012, 4, 854 CAS
.
- E. F. Hare, E. G. Shafrn and W. A. Zisman, J. Phys. Chem., 1954, 58, 236 CrossRef CAS
.
- E. G. Shafrin and W. A. Zisman, J. Phys. Chem., 1960, 64, 519 CrossRef CAS
.
- W. A. Zisman, Advances in Chemistry American Chemical Society, Washington, DC, 1964 Search PubMed
.
- T. Shimogaki, H. Tokoro, M. Tabuchi, N. Koike, Y. Yamashina and M. Takahashi, J. Sol-Gel Sci. Technol., 2015, 74, 109 CrossRef CAS
.
- T. Shimogaki, H. Tokoro, M. Tabuchi, N. Koike, Y. Yamashina and M. Takahashi, J. Sol-Gel Sci. Technol., 2015, 75, 156 CrossRef
.
- http://www.shinetsusilicone-global.com/products/type/silane/detail/k/co_pro.html.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19914j |
| This journal is © The Royal Society of Chemistry 2015 |

