Progress on layered hydrotalcite (HT) materials as potential support and catalytic materials
Thangaraj Baskaran
a,
Jayaraj Christopher
b and
Ayyamperumal Sakthivel
*a
aDepartment of Chemistry, Inorganic Materials and Catalysis Laboratory, University of Delhi, Delhi-110007, India. E-mail: sakthiveldu@gmail.com; Tel: +91-8527103259
bIndian Oil Corporation Ltd, R&D Centre, Faridabad-121007, India
First published on 11th November 2015
Abstract
Mixed metal oxides derived from layered hydrotalcite (HT) materials are extensively used as a potential catalyst and catalytic support in various organic transformations. Numerous such materials have been developed by varying framework divalent and trivalent metal ions, and interlayer anions. The intercalation of anions present in HT facilitates the design of high surface area materials possessing exposed active sites, which enhances the catalytic and adsorption properties. These HT materials have also been used as drug carriers, and in enzyme encapsulation owing to their flexible accommodation of organic anions in the interlayer of the HT structure. The present review is focused on a recent development in the preparation of various HT materials using different metals and intercalating ions and their importance in the various catalytic processes, such as base catalyzed alkylation, isomerization, condensation and esterification. Effectiveness of transition metals containing HT for redox catalytic processes, viz. oxidation of alcohol, alkyl aromatics, phenol and reduction of nitro compounds have been discussed. Further, the importance of the introduction of a hard anion like silicate into the interlayer space of the HT and its applications in various organic reactions has also been covered in this review.
Introduction
Clays are large groups of naturally available versatile materials and are widely used as adsorbents, ion exchangers, catalysts and catalytic support materials.1,2 Clays have a typical layered structure and are broadly divided into two groups, namely cationic (e.g., smectite type) and anionic clay (layered double hydroxide). In cationic clay, the layered structure has two-dimensional oxyanions which are separated by layers of hydrated cations, whereas in anionic clay (e.g. magnesium aluminum-hydrotalcite), the charge on the layer and the gallery ion are reversed with respect to cationic clay.3,4 In comparison to cationic clay, anionic clays are rare in nature, but they are relatively easy and inexpensive to prepare in the laboratory. Hydrotalcite belongs to the class of anionic clays wherein the positively charged layers are formed by the edge sharing of Mg(OH)6 and Al(OH)6 octahedra. Charge balancing anions and water occupy the interlayer spaces.2,5–9The introduction of various inorganic, organic and other anions into the layered HT structure by either post- or in situ synthesis methods widens the scope as catalysts or catalytic supports. High-temperature calcination of HT results in a loss of water and interlayer anions, forming a solid solution of mixed oxide. The resultant mixed oxides or supported HT materials components vary in their molar composition and yield a variety of tailor-made materials that can be demonstrated as potential catalysts, ion-exchangers, adsorbents, corrosion inhibitors, electrode materials and pharmaceuticals.2,10–20 The replacement of carbonate ions in anionic clays is more difficult because of the high charge density, the stability of the resultant HT, and the high affinity of aqueous carbonate with the positively charged cationic sheets.1–25 There are only a few articles focused on the structure of HT, the intercalation of various anions and applications in different fields.2,8,10–14 Reviews on the catalytic applications of these materials are found to be limited. This review article focuses on developments in the preparation of various HT materials and their application as catalyst and catalytic support for various organic transformations.
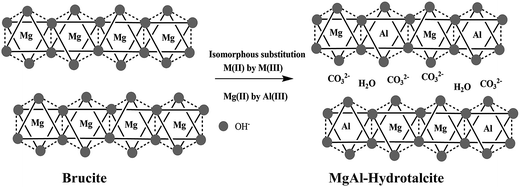
Structural features of hydrotalcite (HT)
The structure of HT materials can be visualized based on brucite sheet. In the layered brucite, Mg2+ ions are surrounded by six hydroxyl ions in an octahedral (Oh) co-ordination and share edges to form infinite sheets. The infinite sheets are stacked upon one another to give a layered network held by hydrogen bonding. If part of the Mg2+ ions is substituted by a trivalent cation having a similar ionic radius, such as Al3+, this results in a positive charge density in the layer (Scheme 1). The electrical neutrality of layered HT materials is maintained by the anions occupying the interlayer positions. Typical anions present in the interlayer are CO32−, NO2−, OH−, and SO42− along with water in hydrated form.9,21–23The general molecular formula of HT-like materials is represented by [M(II)(1−x)M(III)x(OH)2]x+[Ax/nn−]mH2O, where M(II) is divalent (Mg, Ni, Co, Zn) and M(III) is trivalent (Al, Cr, Fe, In) metal cations, x represents the fraction of the M(III) cation, namely x = M(III)/(M(III) + M(II)), and An− denotes anions. The anions present in the interlayer are exchangeable, giving rise to elegant intercalation chemistry.2,5–7 The sheets containing both di- and trivalent cations occupy randomly the octahedral holes of the close-packed configuration of hydroxyl ions and the interlayer constituents; that is, the anion and water are randomly located in this region and possess a high degree of mobility.
There is practically no limitation on the anions that can be intercalated into the interlayer region of the HT-like materials. The natures of the cations that form HTs and the intercalation ability of various anions are summarized in Tables 1 and 2 respectively.
| Inorganic anions | |||
|---|---|---|---|
| Inorganic anions | Reference | Inorganic anions | Reference |
| CO32− | 24 and 25 | V2O74−, VO43−, V10O286− | 37–40 |
| NO32− | 25 and 26 | H2W12O406− | 41 |
| ClO4− | 27 | H2PO4−, HPO42−, PO3−, PO43−, P2O74−, P3O105− | 42 and 43 |
| Halides | 28 | Borate and tetraborate | 44 and 45 |
| SO42−, CrO4−, CrO42−, Cr2O72− | 29 and 30 | MoO42−, Mo7O246− | 46 and 47 |
| OH− | 31 | MnO4− | 48 |
| (AsO4)3− | 32 | OsO42− | 49 |
| Silicate anions | 33–36 | Polyoxometalate anions | 50 and 51 |
| M2+ ion | M3+ ion | References | M2+ ion | M3+ ion | References |
|---|---|---|---|---|---|
| Ca | Al | 76–78 | Ni | Al | 98 |
| Cd | Al | 79 and 80 | Ni | Co | 99 |
| Co | Al | 81 and 82 | Ni | Fe | 94 |
| Cu | Al | 83 | Ni | V | 100 |
| Cu | Cr | 84 and 85 | Zn | Al | 101 and 102 |
| Li | Al | 86 and 87 | Zn | Cr | 103 |
| Mg | Al, Eu | 88 | Co | Co | 104 and 105 |
| Mg | Al | 89–91 | Ni | Ni | 106 |
| Mg | Cr | 92 | Co | Ni | 107 |
| Mg | Fe | 93 and 94 | Co | Fe | 108 |
| Mg | V | 95 | Ni | Fe | 109 |
| Mn | Al | 96 | Zn | Co | 110 and 111 |
| Mg | Mn | 97 | Zn | La | 112 |
Memory effect of HT materials
One of the important properties of hydrotalcite is the memory effect.2,113 During the calcination of HT materials, removal of the interlayer anions, hydroxyl groups and water results in homogeneous mixture of bulk metal oxides. Interestingly, the calcined hydrotalcite is able to regain the original layer structure when it is exposed to anions in aqueous solution. The reforming of the layered structure is controlled by two important factors, namely the choice of starting materials and the temperature of calcination. The starting materials must contain a thermally labile anion, and the temperature of calcination must be controlled so as to avoid excessive heating that would result in the formation of a spinel structure, which is resistant to rehydration. The above memory effect helps to develop a tailor-made variety of materials and is utilized for various applications.2,113–117 These layered materials are relatively less stable at high temperature and are transformed into a homogeneous mixture of solid mixed oxide upon calcination. The uniform distribution of the mixed oxides and homogeneous mixture makes these materials potential catalysts for the various organic transformations.General synthetic procedure
Anionic clays are a large group of natural and synthetic materials readily produced when a suitable mixture of metal salts is treated with, for example, ammonium hydroxide/sodium hydroxide base by the sol–gel method. HT can be synthesized by various methods depending on the specific requirements and properties. The widely accepted methods are co-precipitation, urea hydrolysis, microwave irradiation, hydrothermal and sol–gel processes (Scheme 2). The most common co-precipitation method is used for the preparation of numerous HT materials.118Intercalation on hydrotalcite materials
Intercalation of anions helps to tune the chemical, electronic, optical, and magnetic properties of a host lattice. The intercalation chemistry of HT is quite extensive, which helps to generate a variety of tailor-made materials at ambient conditions. A huge variety of anions (Xn−) are known to have been incorporated (Table 1) into the interlayer region of hosts using a range of methods (Scheme 3).2,5,8,53,119–122 Intercalation of metal-containing anions (viz., polyoxometalate, molybdate, vanadate, chromate) helps the incorporation of a third metal component in the mixed oxide, when it is processed for thermal decomposition. Various characterization techniques have been employed to study the pillaring in HT's and demonstrated how it is possible to follow the process in which the existing interlayer anions are replaced by the pillaring anions, which diffuse into the interlayer region.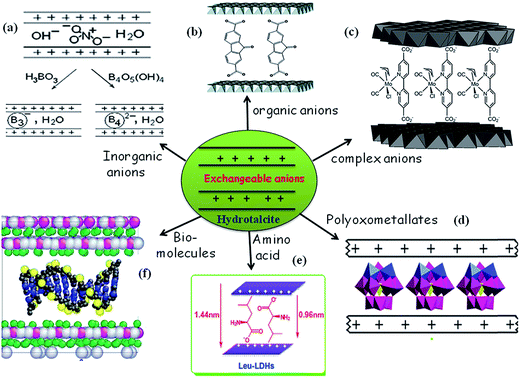 | ||
| Scheme 3 Pictorial representation of various anions intercalated in hydrotalcite materials for various applications ((a) adapted from ref. 44 with kind permission from Elsevier; (b and c) reprinted from ref. 119 and 75 with permission from the Royal Society of Chemistry; (d and e) adapted from ref. 120 and 121 with kind permission from Elsevier; (f) reprinted with permission from ref. 122. Copyright (2008) American Chemical Society). | ||
With intercalation of the bulkier anions (e.g. polyoxometalate, bio-molecules, aromatic anions, complex anions), the basal spacing of the HT increases according to the orientation of the pillaring species in the interlayer, and the crystal grows in the c direction. In many cases, during the direct exchange synthesis of pillared compounds, a partial loss of crystallinity has been observed. These features can be followed using powder X-ray diffraction patterns. In addition, FT-IR studies facilitate the identification of the intercalation of HT, based on the change in vibration mode of interlayer anions such as carbonate, nitrate. The intercalation of various anions facilitates the intrinsic properties of these materials. Similarly, various base catalysts were generated by the introduction of O-t-Bu anion (tert-butoxide), fluoride ion, mixed oxides derived from calcinations, doping of metal ions (La, Ce, Ga, Zr, K, Ca), and a rehydrated form of hydrotalcite.56,114,123–127
Catalytic applications of HT materials
Overviews of HT as a flexible layered material have found it to possess interesting properties for a variety of applications such as base, redox and photo-catalysts, as well as super capacitors, in pharmaceuticals industry and adsorbents.13,19,128,129The present review is restricted to the recent development of HT as a catalyst for organic transformations and application of HT as adsorbents. The ability to synthesize a variety of tailor-made layer materials and their tunable intrinsic properties make these materials promising as catalysts or a catalytic support in many organic transformations (Scheme 4), such as alkylation, isomerization, hydroxylation, transesterification, hydroformylation, redox reactions, condensation and environmentally friendly reactions.
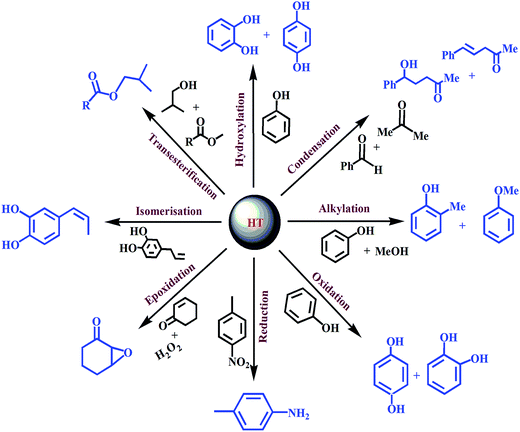 | ||
| Scheme 4 General schematic representation of various organic transformations using HT-based materials. | ||
The following section describes the utilization of such HT materials as catalysts in industrially important organic transformations.
HT materials for carbon–carbon bond formation
Carbon–carbon bond formation is one of the important processes in organic synthesis. In general, C–C formation is achieved by different methods (alkylation, condensation and addition reactions). In particular, alkylation is one of the most significant methods to increase the carbon number. Layered double hydroxides/HT has been known to be efficient catalysts for a variety of C–C bond formation reactions.Alkylation of phenol with alcohols is an industrially important reaction because many of the alkyl phenols are used as raw materials for the synthesis of numerous pharmaceuticals, agrochemicals and other important organic products. Velu et al. studied the alkylation of phenol with 1-propanol and 2-propanol in vapor phase over magnesium aluminum hydrotalcite (MgAl-HT) having Mg/Al ratios of 2, 3 and 4.130 Both O-alkylation and C-alkylation were found in this reaction without any skeletal isomerization of propyl moiety. From the study it was suggested that the reaction proceeded via an SN2 mechanism. Phenol conversion was found to increase with increase in the temperature, and the maximum of 80% was obtained around 350 °C. The MgAl-HT with an Mg/Al atomic ratio of 2 and 3 was found to be suitable in comparison with other catalysts such as CuAl-HT and NiAl-HT. The observed catalytic activity was explained based on strong basicity derived from MgAl-HT in comparison to CuAl-HT and NiAl-HT.
Choudary et al. demonstrated selective 1,4-Michael addition of simple methyl vinyl ketone and acrylate using modified MgAl-HT.131 Simple chalcones were synthesized by donors such as nitroalkane, malononitrile, diethylmalonate, cyanoacetamide and thiols using hydrotalcite in a liquid phase medium under mild conditions. When using HT, it was observed that there were no side reactions such as 1,2-addition and polymerization. The mechanism of this reaction was explained as follows: (i) the abstraction of a proton from the donor gives a carbanion which can be stabilized by the cationic charge of Al in the lattice, (ii) this carbanion further adds to the chalcone to form an enolate, and (iii) the enolate will take a proton from the water to form the final product.
Later, Choudary et al. developed an organo-HT catalyst by incorporating organic chiral molecules L-proline and S-BINOL into hydrotalcite materials.132 This organo-HT was employed for various asymmetric C–C bond formations and the results are reproduced in Table 3.132 Using HT-proline/BINOL employed for aldol and Henry reactions, both reactions demonstrated good products yields of around 95% under mild conditions.
| S. no. | Reactions | Temperature/time (h) | HT-proline (isolated yield %) | HT-BINOL (isolated yield %) |
|---|---|---|---|---|
| 1 | Aldol condensation | RT/24 | 92 | 90 |
| 2 | Nitro-aldol condensation | RT/1, 24 | 98 | 90 |
| 3 | Michael reaction | RT/24 | 40 | 75 |
| 4 | Cyanosilylation | RT/24 | 99 | 99 |
The authors extended this work to study the catalytic behavior for the Michael reaction and cyanosilylation reaction of p-nitro benzaldehyde with trimethylsilyl cyanide. The authors found the advantages of new solid catalysts, with improved catalytic activity of 99% conversion and selectivity under mild liquid phase conditions. Importantly, the organo-catalyst was found to be easy to separate and eco-friendly.
Padmasri et al. studied t-butylation of phenol with iso-butanol using various hydrotalcites obtained by calcination in the temperature range of 350–500 °C.133 Among the various HTs studied, MgAl-HT showed the best conversion at a level of 44.9% conversion with exclusive formation of o-tert-butyl phenol. The basicity of the materials was followed by temperature programmed desorption (TPD) of CO2 and evident in the order of MgO > calcined MgAl-HT (hydrothermal synthesis) > calcined MgAl-HT (co-precipitation) > calcined ZnAl-HT > Al2O3 > calcined MgCr-HT. Similarly, the author also studied acidity by the ammonia TPD method and found the following order of calcined ZnAl-HT > calcined MgAl-HT (co-precipitation) > calcined MgAl-HT (hydrothermal synthesis) > Al2O3 ≈ MgO > calcined MgCr-HT. The better catalytic activity observed on MgAl-HT which was explained based on the presence of the surface basicity and an enhanced acid–base property. The presence of redox chromium species in HT was found to decrease the catalytic activity of the materials and reduce the catalytic activity drastically. The formation of o-tert-butyl phenol was evident as a major product, irrespective of the composition of the HTs.
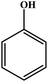
Choudhary et al. studied a novel and efficient route for the synthesis of diaryl ethers by o-arylation of naphthol and phenols with aryl halide using CuFe-HT.134 The results are reproduced and summarized in Table 4. The catalyst showed high activity for the chosen reaction under mild conditions in the absence of externally added bases. Different solvents were studied in the o-arylation reaction, among which DMF was shown to be the best solvent for the chosen coupling reaction, with a maximum conversion of 93%. For the o-arylation of phenol by different aryl halides, the diphenyl ether was yielded at a higher rate using iodobenzene. Further, the author found that the catalyst was reusable for several times without loss of any activities with the product yield of 88%.
| Entry | Aryl halide | Phenol | Time (h)/temperature (°C) | Conversion (%) | Product |
|---|---|---|---|---|---|
| a Reaction conditions: 1 mmol aryl halides = 1.2 mmol phenols; 3 mL: solvent (DMF); 50 mg: catalyst. | |||||
| 1 | 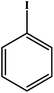 |
 |
7/140 | 93 | 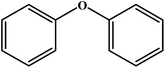 |
| 2 | 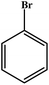 |
 |
10/140 | 88 | 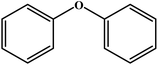 |
| 3 |  |
 |
15/140 | 22 |  |
| 4 |  |
 |
15/140 | — | No product |
| 5 |  |
 |
8/140 | 93 | 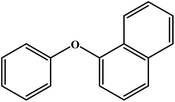 |
Climent et al. studied the Claisen–Schmidt condensation of benzaldehyde with substituted acetophenones over base catalysts like MgAl-HT and Cs exchanged zeolite sepiolite.135 The best activity was observed on MgAl-HT having Al/(Al + Mg) ratios between 0.25 and 0.30. The authors found that the increase in Al content was caused by segregation of Al on the surface, resulting in the generation of cation defect sites of less coordinated oxide ions. Further, it was evident from their studies that the crystallite size plays a major role on the catalytic activity. The smaller crystallite size (<50 nm) was found to be more active. In addition, it was proposed that the higher population of lower coordinated oxide ions served as active sites for the Claisen–Schmidt condensation.
Choudary et al. performed nitroaldol condensation (Henry reaction) for the first time with activated MgAl-HT in a liquid phase medium under mild conditions.56 The reaction was studied with benzaldehyde and nitromethane using modified hydrotalcite (activated at 450 °C). The results were compared with a variety of solid bases such as sodium hydroxide, neutral aluminum oxide, magnesium oxide and diamino-functionalized MCM-41 under similar conditions. The results are reproduced in Table 5. Basic hydroxyl sites present in the modified HT favoured nitroaldol reactions with a good yield (95%) in a short time. The catalyst was found to be non-toxic, inexpensive, with high catalytic activity under mild liquid phase conditions and zero emission of pollutants.
| S. no. | Catalyst | Time (h) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: nitromethane (10 mmol), benzaldehyde (2 mmol), 0.2 g of catalyst; room temperature. | |||
| 1 | Modified MgAl-hydrotalcite | 0.5 | 95 |
| 2 | Aluminium oxide | 12 | 37 |
| 3 | Magnesium oxide | 8 | 51 |
| 4 | NaOH with PTC | 2 | 70 |
| 5 | Diamino-functionalised MCM-41 | 3 | 97 |
Tichit et al. examined aldol condensation of acetaldehyde with heptanal using different types of solid base catalysts in a liquid phase medium.136 Several reaction parameters such as temperature, substrate molar ratio and solvent were investigated. The observed higher conversion (more than 80%) was explained by cross- and self-condensation of the aldehydes on basic and acid-pair sites. Stronger Lewis basic sites of MgO or Brönsted-type basic sites of the rehydrated mixed oxide tend to favor the formation of carbanion from heptanal. The resulting carbanion leads to cross-condensation with acetaldehyde, and subsequently self-condensation leads to the 2-pentyl-2-butenal and 2-pentyl-2-nonenal respectively. The most efficient material for the cross-condensation to 2-nonenal was found to be MgAl-HT obtained by calcination at 600 °C. A maximum yield of 21% was observed at 120 °C in ethanol as solvent with an acetaldehyde to heptanal ratio of 2. The authors also found that an excess of acetaldehyde is necessary to achieve a good balance between both reactants in the adsorbed state and then to favor the cross-condensation.
Sharma et al. investigated aldol condensation of propyl alcohol using different catalysts in the liquid phase condition under solvent-free conditions.137 The maximum conversion (97%) was observed over MgAl-HT with an Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio of 3.5 at 100 °C for 10 h with selective formation of 2-methylpentanal. Among the other catalysts used, such as alkali ion-exchanged zeolites, alumina, alkali-treated alumina and calcined MgAl-HT showed better conversion. Except for MgAl-HT, all the other catalysts are weak bases and the alkali ion-exchanged zeolites basicity depends on the nature of the cations. In the calcined MgAl-HT materials (calcined at 450 °C), the resulting formation of homogeneously dispersed mixed oxides of Mg and Al with strong Lewis basic sites (Mg2+–O2− pairs) and weaker basic sites (OH-groups) was found to be responsible for higher catalytic activity.
Al molar ratio of 3.5 at 100 °C for 10 h with selective formation of 2-methylpentanal. Among the other catalysts used, such as alkali ion-exchanged zeolites, alumina, alkali-treated alumina and calcined MgAl-HT showed better conversion. Except for MgAl-HT, all the other catalysts are weak bases and the alkali ion-exchanged zeolites basicity depends on the nature of the cations. In the calcined MgAl-HT materials (calcined at 450 °C), the resulting formation of homogeneously dispersed mixed oxides of Mg and Al with strong Lewis basic sites (Mg2+–O2− pairs) and weaker basic sites (OH-groups) was found to be responsible for higher catalytic activity.
Faba et al. studied aqueous phase aldol-condensation of acetone and furfural using three different catalysts: MgZr, MgAl and CaZr-HT.138 The catalytic activity and selectivity were correlated with their physico-chemical properties. It was observed that MgZr oxide showed the best performance with 60% for C13 product, followed by the MgAl (43%) and CaZr (15.5%). MgAl and MgZr gave similar yields of more than 74%. However, C8 products formation was more favorable on MgAl oxide, due to the strong influence of the retro-aldolization reaction. The efficiency of the catalyst was decreased in the order of MgZr > MgAl > CaZr.
Bharali et al. investigated nitroaldol condensation using mixed oxides derived from different HT materials.139 The authors prepared catalysts such as CoMgAl-HT and NiMgAl-HT by partial substitution of Mg2+ with Co2+ and Ni2+ metal ions, keeping the (M2+Mg/Al) ratio of 3. The metal ions substitution affects the crystallization, as was observed from the XRD patterns. The as-synthesized and calcined materials were tested for nitroaldol condensation under solvent-free conditions and the results are reproduced in Table 6. Among the various HT catalysts calcined, NiMgAl-HT showed better conversion than the other as-synthesized and calcined HT materials. The observed higher conversion was explained based on the greater surface area and pore volume on NiMgAl-HT. Further, the calculated TOF for cal-NiMgAl-HT was found to be superior to other catalysts and yielded similar activity in a three-recycle study.
| S. no. | Catalyst | Time (h) | Conversion (%) | TOF (h−1) | Textural properties | |
|---|---|---|---|---|---|---|
| BET surface area (m2 g−1) | Pore volume (cm3 g−1) | |||||
a Reaction conditions: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (4-nitrobenzaldehyde 10 (4-nitrobenzaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitromethane (1 mmol)); 10 mg catalyst; room temperature. nitromethane (1 mmol)); 10 mg catalyst; room temperature. |
||||||
| 1 | MgAl-HT | 12 | 86 | — | — | — |
| 2 | NiMgAl-HT | 8 | 91 | — | — | — |
| 3 | CoMgAl-HT | 10 | 84 | — | — | — |
| 4 | Cal-MgAl-HT | 6 | 95 | 12.5 | 561 | 0.97 |
| 5 | Cal-NiMgAl-HT | 2 | 99 | 149 | 753 | 1.27 |
| 6 | Cal-CoMgAl-HT | 4 | 97 | 36 | 401 | 0.93 |
Overall in the above section summarized the importance of hydrotalcite materials on C–C bond formation viz., alkylation, condensation and addition reactions. For example MgAl-HT material possessing strong basicity favors the better conversion on aromatic alkylation without any skeletal isomerized products. Further, MgAl-HT with variable Mg/Al ratio able to tune the activity on condensation, addition and coupling reactions. In particular Michael addition reaction, the materials yielded desired products without formation any polymerized products. Various form of hydrotalcite materials having different metal ions and interlayer anions showed better yield on a variety of condensation reactions. Metal oxides derived from MgAl-HT produce strong Lewis basicity (Mg2+O2−) and weak basic sites (OH groups), which is responsible for the better activity on various reactions.
Hydrotalcites as redox catalysts
Oxidation and reduction are fundamental reactions in organic synthesis. A variety of HT or supported HT materials have attracted considerable attention in the recent decade as heterogeneous redox catalysts.140Choudary et al. tried hydroxylation of alkene using different catalysts like OsO42− exchanged HT, SiO2-HT and resin-OsO4.142 An active osmium catalyst was readily prepared from non-volatile K2OsO4·2H2O by simple ion-exchange techniques. Hydroxylation was examined using trans-stilbene as a model compound in various reaction conditions. When trans-stilbene was added to a mixture of HT-OsO4, chiral ligand and NMO, the desired diol product was obtained in 96%. Mono- and tri-substituted olefins also yielded diols with maximum selectivity.
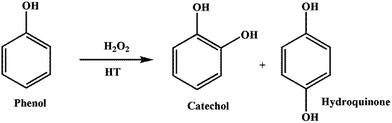
Phenol to catechol and hydroquinone is an important industrial process (Scheme 5) and these products are used in various fields. Antonyraj et al. studied phenol hydroxylation using various hydrotalcite (HT) materials (as-synthesized and calcined) with H2O2 as oxidant.143 Among the various HT materials (CuZnAl-HT, CuMgAl-HT, CuNiAl-HT and CuCoAl-HT), CuZnAl-HT was observed to give the best phenol conversion in both as-prepared and calcined form (21.6 and 17.6% respectively) and the results are reproduced in Table 7.143 The authors explained that the better activity is based on the presence of Cu2+ in the octahedral sites of HT materials.
| S. no. | Catalysts | As-synthesized | Calcined | ||
|---|---|---|---|---|---|
| Conversion (%) | CAT/HQ | Conversion (%) | CAT/HQ | ||
| a Reaction conditions: phenol: 1 g, catalyst: 10 mg, oxidant (H2O2): 0.6 mL, water: 10 mL, T = 65 °C, t = 2 h; CAT/HQ-catechol and hydroquinone ratio. | |||||
| 1 | CuZnAl-HT | 21.6 | 1.9 | 17.6 | 1.8 |
| 2 | CuMgAl-HT | 11.4 | 9.7 | 0.3 | ∞ |
| 3 | CuNiAl-HT | 2.7 | 26.5 | 2 | 2.4 |
| 4 | CuCoAl-HT | 1.4 | 0.75 | 1 | 1.1 |
Friedrich et al. obtained hydroxylation of alkene into diols selectively using Os/Cu-HT with N-methyl morpholine oxide (NMO) as co-oxidant.144 It was observed that there was no activity of alkene with the use of only oxidant viz., NMO, which proved that NMO is not the primary oxidant. The mechanism was explained as follows: HT activates the oxygen of the NMO, and the resulting activated NMO oxygen reacts with olefins to yield diols. Os/Cu-HT shows better conversion and selectivity in diols synthesis.
Kaneda et al. carried out oxidation of allylic and benzylic alcohols to the corresponding aldehydes or ketones using Ru-MgAl-HT in the presence of molecular oxygen.146 The conversion and yield were poor with the MgAl-HT. Oxidation of allylic and benzylic alcohols was efficiently catalyzed by ruthenium-HT and yielded corresponding carbonyl compounds. The active sites of Ru-hydrotalcite might be derived from hydroxyl groups associated with ruthenium cations. It was speculated that alcohols coordinated at metal centre followed by interaction with hydroxyl group present in ruthenium species, and the ligand exchange reactions between the hydroxyl and alcohol give a ruthenium-alkoxide species which undergoes β-elimination to afford an aldehyde product.
Matsushita et al. studied the oxidation of cinnamyl alcohol and various other (aliphatic, aromatic and heterocyclic)alcohols using Ru-containing HT in the presence of molecular O2.147 A series of hydrotalcites such as Ru-CoAl-HT, Ru-MnAl-HT, Ru-FeAl-HT, Ru-ZnAl-HT and Ru-MgAl-HT were investigated. Ru-CoAl-HT yielded complete conversion within 40 min and the results are shown in Fig. 1. Ru(VI) to Ru(IV) induced by the introduction of Co cations was shown to be responsible for oxidation of alcohol. The catalyst was also found to be suitable for oxidation of various other alcohols, and it was further demonstrated that the catalysts are recyclable.
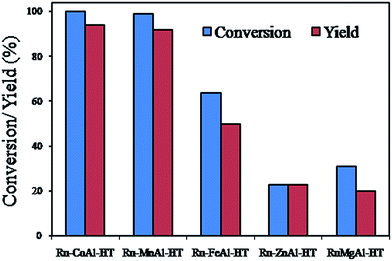 | ||
| Fig. 1 Oxidation of cinnamyl alcohols using various Ru-hydrotalcites (reproduced from ref. 147, with permission from the Royal Society of Chemistry). | ||
Sn-doped HT materials were prepared and studied for Baeyer–Villiger oxidation of ketones by Pillai et al.148 Ion exchange and the wet impregnation method were used for preparation of Sn loading on HT. Different concentrations of 0.4, 1.5 and 3 wt% of Sn were loaded on calcined MgAl-HT and used for the oxidation reaction. The authors observed a maximum conversion of 58%, with more than 95% selectivity of lactones using a 1.5 wt% Sn loaded sample. They found that ion-exchanged catalyst showed better activity than wet impregnated catalyst, and explained this based on the ion-exchange method producing a homogeneous distribution of Sn and creating a Lewis acid center. The observed high activity on oxidation of ketones was attributed to the presence of active Sn sites in the interstitial position of the HT support materials. The authors also explained a possible mechanistic way as follows: Sn sites activate the carbonyl group of ketones, followed by nucleophilic attack by the peroxide species to form Criegee adduct, which rearranges to give the product lactone.
Kirm et al. carried out epoxidation of styrene using HT materials with hydrogen peroxide and acetonitrile as oxidant.149 It was explained that basic hydroxyl groups of hydrotalcite abstract hydrogen from H2O2 to form a per-hydroxyl anions, which nucleophilically attack the nitrile, to generate peroxycarboximidic acid,149 and was found to be active intermediate oxidant. Acetone and water was used as solvents. The HT obtained with the Mg/Al mole ratio of 4 yielded the maximum of 88% with exclusive formation of styrene oxide. HT having Mg/Al ratios higher than 4 was found to possess a surface layer of amorphous magnesia, which reduced the catalytic activity. In the absence of acetonitrile, no activity was observed. It was also observed that solvents play an important role in improving the catalytic activity.
Kantam et al. synthesized a catalyst consisting of rac-BINOL (racemic 1,1′-2-naphthol) in CuAl-hydrotalcite (CuAl-HT) for the alcohol oxidation as a green process.150 The prepared different ratios of CuAl-HT consisting of BINOL and used them for various types of alcohol oxidation in the presence of different bases. The best results were evident with K2CO3. The employment of a chelating ligand used in the study provided the major driving force behind the evaluation of the active metal catalysts which activate the molecular oxygen in the oxidation process. The developed Cu/Al-rac-BINOL was shown to be an efficient and re-usable catalyst for oxidation of benzylic, secondary, heterocyclic alcohols to the corresponding oxidized products under mild conditions.
Mitsudome et al. found that hydrotalcite (HT)-supported gold nanoparticles (Au/HT) are a highly efficient heterogeneous catalyst for the aerobic oxidation of alcohols under mild conditions.151 The catalyst was also effective towards less reactive cyclohexanol derivatives to produce corresponding cyclohexanone. The product increased with decrease in the particle size of the Au nanoparticles. In other words, a small particle size was essential for effective alcohols oxidation. The use of other metal particles such as Pd, Ru and Ag resulted in lower activity under similar reaction conditions.
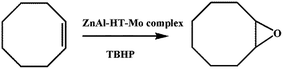
Gomes et al. developed a catalyst by Mo-complex intercalated ZnAl-HT for epoxidation of olefins (Scheme 6).75 The catalyst was prepared by intercalating anions [Mo(η3-C3H5)(CO)2Cl(bpdc)]2− (bpdc = 2,2′-bipyridine-5,5′-dicarboxylate) by ion exchange on ZnAl-HT. The intercalation of anions was confirmed using FT-IR spectra, from which the author detected the presence of bands at 1800–2020 cm−1 corresponding to carbonyl group in Mo(CO)2. The observed increase in the interlayer distance to 18.3 Å on the (003) plane was due to the presence of bpdc anion perpendicular to the host lattice. The epoxidation was carried out using cis-cyclooctene and TBHP (tert-butylhydroperoxide) in decane as oxidant in a liquid phase medium. The catalyst showed the conversion of cyclooctene to cyclooctene oxide with a maximum of 96% at 24 h. The authors mentioned that the better catalytic activity observed was due to active Mo species and further mentioned the presence of a host–guest system, proposed as a suitable catalyst for the field of bio-medicine.
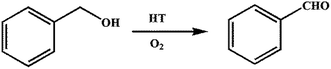
Dong et al. prepared rehydrated HT materials and studied them for aerobic oxidation of benzyl alcohol to benzaldehyde (Scheme 7).152 Various catalysts were prepared by the co-precipitation method, and the materials were calcined and rehydrated for catalytic study. Rehydrated MgAl-HT support promoted the catalytic activity of Co3O4. Rehydrated Co3O4/HT was observed to yield 74% benzyl alcohol, and was compared with calcined CoMgAl-HT (21%). Among the various transition metals ions, the Cu promoted HT showed better catalytic activity than Fe or Zn based catalyst. The authors found that rehydrated HT with the molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg
Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al (1
Al (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed the maximum benzyl alcohol conversion (92%) and with 75% selectivity of benzaldehyde.
1) showed the maximum benzyl alcohol conversion (92%) and with 75% selectivity of benzaldehyde.
Lv et al. studied ethylbenzene oxidation using modified HT materials under solvent-free conditions.153 The modified HT catalyst was prepared as follows: first MgAl-HT was prepared by the co-precipitation method and calcined, the calcined HT being denoted as LDO. As the subsequent step, calcined LDO was silylated with mercaptopropyltrimethoxysilane (MPTS) followed by MnO2 was intercalated using KMnO4 and represented as LDH-Si(SH)–Mn. Silylation was confirmed by the FT-IR spectra, where the authors observed the presence of two bands (2930 and 2850 cm−1) corresponding to asymmetric and symmetric stretching vibration of –CH in the MPTS. Further, the formation of Mn oxides was evident from the XPS spectrum, which showed Mn 2p1/2 and Mn 2p3/2 peaks at 653.4 and 642.2 eV respectively. The above prepared catalyst was tested for oxidation of ethylbenzene using O2 as oxidant and TBHP as initiator in a liquid phase medium. No appreciable conversion was observed in the absence of catalyst and initiator. The use of LDH-Si(SH)–Mn showed a maximum conversion of around 29.5% with the acetophenone selectivity of 95% evident. The authors also observed that the prepared Mn-containing silylated MgAl-HT was heterogeneous in nature and without any leaching of the active species during the reaction.
Dixit et al. synthesized MgAl-HT and MgAl-mixed oxide supported copper catalyst for dehydrogenation of benzyl alcohol.154 Copper finely dispersed on MgAl-HT support, which found to influence the surface acidic, basic and reducibility properties. The catalyst without copper was found to be inactive. Cu-HT shows 94% conversion towards benzaldehyde, and was found to be comparable with conventional commercial copper oxide catalyst. The mechanistic aspect was explained as follow: the acidic and metal sites proposed as adsorption sites for the alcohols and the basic sites extract a proton from the alcoholic hydroxyl group, forming metal alcoholate species on the catalyst surface. The β-hydride elimination with the help of metal ion-Lewis acid sites resulted in the carbonyl product.
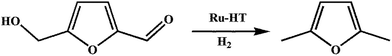
Nagpure et al. applied Ru-hydrotalcite (HT) for selective hydrogenolysis (Scheme 8) of 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF).155 Ru-doped MgAl-HT was prepared by the co-precipitation method and the pH was maintained in the range of 9.5–10. Different concentrations of Ru were doped in the MgAl-HT materials and represented as RH-1 (0.56 wt% Ru), RH-2 (1.0 wt% Ru), RH-3 (1.7 wt% Ru) and RH-imp (0.58% Ru impregnated). The authors screened different parameters on the catalytic activity and found the best condition of 220 °C, using 10 bar H2 pressure, 2-propanol and 50 mg HT. Among the various Ru-HT materials, RH-1 (0.56 wt% Ru) showed the best catalytic activity (100 mol% HMF conversion) with a selectivity of DMF (58 mol%).
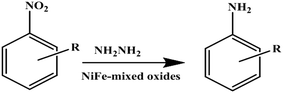
Shi et al. prepared nickel–iron mixed oxide from the corresponding HT materials and used as catalyst for reduction of nitroarenes under mild conditions (Scheme 9).157 Various nitro-arenes with different substituted groups were successfully reduced using hydrazine hydrate over NiFe-HT to corresponding amines. The desired product was not observed in the absence of either nickel–iron metal oxide or hydrazine hydrate. It was clearly demonstrated that both nickel–iron metal oxide and hydrazine hydrate played an important role in the reduction of nitro-arenes. In addition, nickel–iron mixed oxide showed excellent activity for the reduction of sulfur-containing aromatic nitro compounds. Chemo-selective reduction was evident in this reaction. The author explained that the catalytic system described might be a promising alternative to sulfide reduction and Fe/HCl reduction, which are currently widely used for preparing sulfur-containing aromatic amines in industry.
Sangeetha et al. prepared Pd-supported MgAl-HT and tested for hydrogenation nitrobenzene in a vapor phase medium.158 The MgAl-HT was prepared by the standard co-precipitation method and 1 wt% Pd was wet impregnated on calcined HT using palladium chloride acidified with HCl. The final Pd-supported HT material was calcined at 450 °C before being used for catalytic activity. The catalytic activity of the materials was tested at different temperatures and also compared with 1 wt% PdMgO and 1 wt% γ-Al2O3 under identical conditions. The authors observed decrease in the rate of conversion of nitrobenzene to aniline with increase in temperature and this has been explained as being due to coke formation and poisoning due to the generation of water. It was observed that 1 wt% Pd-HT showed better activity than 1 wt% Pd–MgO and 1 wt% Pd-γ-Al2O3 and all cases 98% aniline was observed as the major product. The homogeneously distributed Pd particles on HT materials were responsible for the better activity observed.
The flexible framework of hydrotalcite facilitates to incorporate various transition metal ions in the framework and shown as potential redox catalysts for various organic transformations. The presence of divalent copper (Cu2+) metal ion specially favored the selective synthesis of catechol and hydroquinone from phenol. Similarly Ru, Au and Sn doped HT used for oxidation of various aliphatic, aromatic and cyclic alcohols into corresponding aldehydes, ketones and acids. The introduction of Ni on HT framework facilitates the reduction of aromatic, conjugated aldehydes and heterocyclic aldehydes. Further, HT showed promising support for homogenous distribution of metal ions like Pd, Ni, Fe etc. and used for selective reduction of nitro compounds.
Transesterification
The increasing demand for energy and greater environmental awareness has prompted much research towards the production of alternative fuel using environmentally acceptable routes. Biodiesel is an interesting alternative energy source owing to the product's favorable effects on the environment.159,160Choudary et al. carried out transesterification of β-keto ester using t-butoxide anion exchanged MgAl-HT, MgAl-Ot-but-HT, which was prepared from MgAl-HT with potassium t-butoxide in THF at room temperature.161 The transesterification of normal and β-keto esters was carried out with a variety of primary, secondary, unsaturated, allylic, cyclic and hindered alcohols for the first time and showed a maximum yield of 98% obtained on MgAl-Ot-but-HT with the fastest reaction rate. Pure calcined MgAl-HT showed a moderate yield even at longer duration.
Zeng et al. studied the transesterification of rapeseed oil using HT obtained with different molar ratios of Mg and Al.162 The ester formation was increased up to 90% in 4 h with a reaction temperature of 65 °C. Further, the reaction profiles indicated that the ester conversion increased with increase of the amount of catalyst from 0.5 to 1.5%. However, the conversion decreased with further increase of the amount of catalyst, which was explained based on the difficulty of achieving a homogeneous reaction and catalyst mixture.
Wang et al. prepared Mg2+, Fe3+, Al3+ containing hydrotalcite for the transesterification reaction of soybean oil with methanol to produce biodiesel.163 Different molar ratios of Mg/Fe-HT were prepared and studied for the above reactions. The conversion of FAME (fatty acid methyl ester) was increased with Mg content in Mg/Al/Fe-HT. It was confirmed that the addition of a small amount of Fe3+ in the Mg/Al hydrotalcite enhances the conversion of FAME. However, the conversion of FAME decreased during recycle studies. This was explained based on the metal ions leaching into solution while washing the catalyst.
Yadav et al. made an attempt to convert glycerol to glycerol carbonate using calcined MgAl-HT/hexagonal silica (CHT-HMS) materials, as one of the alternatives to valorize glycerol.164 The authors confirmed the hexagonal silica in the material using FT-IR spectra, which showed a band around 1083 cm−1 corresponding to asymmetric stretching of Si–O–Si bonds. The reaction was performed in an autoclave reactor by charging glycerol, dimethyl carbonate and methanol (solvent) and the reaction is shown in Scheme 10. The authors found that MgAl-HT obtained with an Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio of 2
Al ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 yielded a maximum glycerol conversion of around 84.7% with selectivity of glycerol carbonate (84.3%). Further, the reaction was found to be second order with activation energy of 12.56 kcal mol−1. No conversion was observed in the presence of pure HMS under identical reaction conditions. The better catalytic activity was explained based on the higher surface area, uniform pore size and bi-functional characteristics of CHT-HMS.
1 yielded a maximum glycerol conversion of around 84.7% with selectivity of glycerol carbonate (84.3%). Further, the reaction was found to be second order with activation energy of 12.56 kcal mol−1. No conversion was observed in the presence of pure HMS under identical reaction conditions. The better catalytic activity was explained based on the higher surface area, uniform pore size and bi-functional characteristics of CHT-HMS.
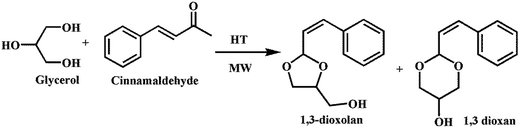
Glycerol is a major by-product in bio-diesel synthesis, which was converted into a value-added product by acetalization of glycerol (Scheme 11) using hydrotalcite (HT) materials by Prakruthi et al.165 All the hydrotalcite materials, namely ZnAl-HT, MgAl-HT, CuAl-HT and CoAl-HT, were synthesized by the co-precipitation method and tested for catalytic activity. The reaction was carried out in a microwave (MW) reactor by charging with glycerol (Gly) and cinnamaldehyde (CA). Among the various HT materials, MgAl-HT was found to have the best catalytic activity (conversion 56%) with 96% selective formation of 1,3-dioxolan (5-membered cyclic acetal). The details of the catalytic activities of various HT materials are summarized in Table 8. The authors observed that rehydrated MgAl-HT showed better activity (77% conversion) with 96% selectivity of 1,3-dioxolan, which was explained based on the formation of medium and strong basic sites by redistribution of OH groups on MgAl-HT materials.
Heterogeneous catalysts are growing interest in bio-diesel production by transesterification process. Higher basicity of various anions intercalated HT or mixed oxides derived from parent HT materials shown potential applications in transesterification. Various organic compounds like β-keto ester, poultry fat, rapeseed oil, and soybean oil etc., used for transesterification with low molecular weight alcohols such as methanol. In addition HT materials showed potential for conversion of glycerol into value added products such as glycerol carbonate and glycerol acetal compounds. In particular, rehydrated MgAl-HT yielded better conversion on glycerol acetylation, which was explained based on the redistribution of OH groups on MgAl-HT and formed medium-to-strong basic sites. The HT possessing transition metal ions further enhances the conversion FAME (fatty acid methyl ester).
Other organic reactions
C–C double bond isomerization is of great interest because of its potential commercial applications for the synthesis of fine, perfumery chemicals and in the pharmaceutical industries.16,166 Kishore et al. studied the isomerization of safrole to corresponding thermodynamically stable iso-safrole using different hydrotalcite materials as the base catalyst.167 The isomerization of safrole reaction was carried out in a batch reactor using different MgAl, MgFe, MgCr-HT and the activity was also compared with Mg(OH)2 and Al(OH)3 (Table 9). The activity of HT prepared with Mg/Al at molar ratios of 2 and 3 showed a poor yield; however, the maximum conversion of around 75% with cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratios around 15
trans ratios around 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 was obtained over MgAl-HT having an Mg/Al ratio of 6. trans-Isomer was formed predominantly in all catalysts due to its better thermodynamic stability. Further, the conversion was found to increase with increase in the polarity of the solvent. The conversion and selectivity of iso-safrole decreased in the following order: MgAl-HT ∼ MgFe-HT > MgCr-HT. This was possibly due to the lower thermal stability of the Fe and Cr systems compared to Al.
85 was obtained over MgAl-HT having an Mg/Al ratio of 6. trans-Isomer was formed predominantly in all catalysts due to its better thermodynamic stability. Further, the conversion was found to increase with increase in the polarity of the solvent. The conversion and selectivity of iso-safrole decreased in the following order: MgAl-HT ∼ MgFe-HT > MgCr-HT. This was possibly due to the lower thermal stability of the Fe and Cr systems compared to Al.
| S. no. | Catalyst (ratio of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al) Al) |
Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|
| cis-Isomer | trans-Isomer | |||
| a Reaction conditions: substrate: 0.5 g, DMF: 20 mL, catalyst: 250 mg, temperature: 200 °C, time: 6 h. | ||||
| 1 | MgAl-HT (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2 | — | 100 |
| 2 | MgAl-HT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1 | — | 100 |
| 3 | MgAl-HT (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
28 | 11 | 89 |
| 4 | MgAl-HT (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
75 | 15 | 85 |
| 5 | MgAl-HT (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
33 | 15 | 85 |
| 6 | MgAl-HT (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
54 | 15 | 85 |
| 7 | Mg(OH)2 | 0.34 | — | 100 |
| 8 | Al(OH)3 | 0.11 | — | 100 |
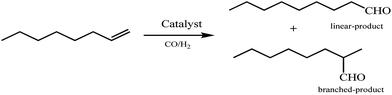
Rh-complex impregnated HT was prepared and utilized for synthesis of aldehyde and alcohol in an eco-friendly manner by Sharma et al.168 Rhodium complex HRh(CO)(PPh3)3 was impregnated on the surface of the HT and the materials were investigated for synthesis of aldehyde and alcohol from propylene. Solid state 31P NMR confirms that three phosphorous atoms possess the same environment in an equatorial position, and H− and CO are in the axial position trigonal bipyramidal in the complex structure. Mg/Al-HT with a molar ratio of 3.5 showed increased selectivity for 2-ethyl hexanol with an increase in the amount of catalyst. The enhancement in the catalytic activity was explained based on the increased basicity of the catalyst. The amount of Rh-complex in the catalyst significantly influenced the selectivity of 2-ethylhexanol.
Delidovich et al. systematically studied the isomerization of glucose into fructose using MgAl-HT.169 Different MgAl-HT catalysts were prepared by varying the synthesis parameter and analyzed using various techniques. MgAl-HT was prepared in four different ways using the co-precipitation method as follows: (i) HT prepared at 150 °C aging in an autoclave-denoted as HTs-150, (ii) HT prepared at pH 9.0–9.5 at room temperature aging-denoted as HTs-9.5-RT, (iii) HT prepared at pH 10.0–10.2 at room temperature aging-denoted as HTs-10-RT, and (iv) HT prepared in ethanol–aqueous medium at pH 9.0–9.5 at room temperature aging-denoted as EHTs-RT. The authors found that the preparation method influences the basicity of the materials and the increasing order is as follows: HTs-150 < HTs-9.5-RT < HTs-10-RT ≈ EHTs-RT. The reaction was performed in an autoclave reactor in a nitrogen atmosphere and found the maximum glucose conversion (30%) using EHTs-RT with fructose selectivity (87%). Based on their studies, it was concluded that EHTs-RT having uniform dispersed primary particles with small crystallites yielded better activity than HT prepared in an aqueous medium.
HT materials as potential support for environmental applications
In recent times, the level of pollutants has been increasing day by day in aqueous environments and potential adsorbent is scarce. HT and the materials derived from it possess a strong anion exchange capacity and can be used as a potential adsorbent. Hydrotalcite materials have been exhibited as potential sorbents in water purification and the treatment of effluent streams from wastewater processes.13,170Inacio et al. tested the adsorption characteristics of the herbicide MCPA (4-chloro-2-methylphenoxyacetic acid) on hydrotalcite under laboratory conditions.171 MgAl-HT materials possessing different anions such as Cl−, NO32− and CO32− were used for adsorption studies. After the adsorption, the authors detected from X-ray diffraction analysis that there was an enlargement of the basal spacing (d(003) = 2.21 nm) on [Mg3Al–Cl] and [Mg3Al–NO3], indicating that some of the internal chloride/nitrate anions were exchanged by MCPA. It was demonstrated that HT-like compounds display interesting adsorption properties with regard to anionic MCPA. The adsorption was caused by an anion exchange mechanism involving the whole solid phase. At low concentrations of MCPA, only the exchange of surface sites was involved, while an internal exchange occurs for high ratios of MCPA/HT. It was described that various factors affect adsorption properties, which are the pH of the solution, the charge density of the layers, the anion exchange capacity, the nature of the starting anions, and the morphology of the adsorbent.
Orthman et al. prepared HT materials to investigate their adsorption capabilities in the removal of coloured organic substances from various aqueous systems.172 Different molar ratios of Mg/Al-HT were prepared for the removal organics. Six dyes were used throughout the experiments: Acid Blue 29, Basic Blue 66, Reactive Blue 4, Disperse Red 1, Eosin B and Basic Blue 9. These dyes were used without any further purification and a reference solution was prepared at the time of each adsorption experiment. The authors explained the adsorption process as being based on two possible mechanisms that were established: (i) surface adsorption and (ii) anion exchange. It was explained that the removal of dye followed the mechanism path of adsorption followed by anion exchange.
Gillman prepared clay material like hydrotalcite (HT) for the removal of arsenite (As(III)) and arsenate (As(V)) from drinking water.173 Different anion-containing hydrotalcites viz., HT-carbonate, HT-nitrate and HT-chloride, were prepared for the arsenic removal study. The HT materials were found to reduce arsenic concentrations to acceptable levels, when arsenic-containing water passed through the HT. The stabilized HT granules were able to reduce the relatively high concentration of As to an acceptable level, in practice, below 10 μg L−1. Most probably, even lower concentrations in the filtrate could be achieved by reducing the percolation rate or increasing the amount of Cl-HT in the filter pouch. The best condition was established using an apparatus with a smaller diameter and greater path, whose length would allow greater contact time between the water and HT, and facilitate the effective removal of arsenic.
Núñez et al. prepared Mg, Co, Ni-HT and its calcined materials were studied for fluoride removal from the aqueous system.174 The calcined Mg-HT, Co-HT, Ni-HT were treated with fluoride solutions in batch systems, and F− ions in the remaining solutions was determined using a fluoride ion selective electrode. The calcined Mg-HT was regenerated to its original crystalline structure after coming into contact with fluoride ions, whereas Ni and Co-HT remained as oxide mixtures. The calcined Ni-HT shows the best characteristics for fluoride ions sorption from aqueous solutions.
Sampieri et al. studied the degradation of methyl parathion (MP) using hydrotalcite (HT) materials, MP being a very toxic organophosphate pesticide.175 Depending on the basic strength, some calcined HT can catalyse the transformation of MP to p-nitrophenol (p-NP). Mg-HT, Zn-HT and Ni-HT were used for this degradation process and the degradation was monitored using UV-absorption techniques. UV-Vis spectra for MP solution stirred with Mg-HT, Zn-HT and Ni-HT mixed oxides clearly showed that MP can be identified by the intense and broad peak at 270 nm and a peak around 410 nm for p-NP. The authors found that using Mg-HT within 1200 minutes yielded complete degradation of MP to p-NP. Zn-HT and Ni-HT showed less effective fractional degradation of MP. The degradation of MP with hydrotalcite oxides was explained based on the heterogenization of a homogeneous catalysis mechanism that depends on the basic strength of the calcined HTs.
Anirudhan et al. prepared 4-ethyl thiosemicarbazide intercalated hydrotalcite (HT) materials for the removal of uranium (U(VI)) and thorium (Th(IV)) from waste water.176 FT-IR spectra confirmed the formation of complex-HT, with the appearance of a new vibrational band around 1220 cm−1 corresponding to thiocarbonyl group. A batch adsorption study proved that the new adsorbent, ethyl-thiosemicarbazide intercalated hydrotalcite, has a high adsorption capacity of U(VI) and Th(IV) ions in the pH range of 4–6. This result demonstrates that HT-composite materials can be effectively used for the removal of heavy metal ions from aqueous solutions.
Pahalagedara et al. carried out an experiment to remove azo dyes (RBV-5r) using NiAl-HT materials.177 Co-precipitation followed by the ultrasound irradiation method was used for the preparation of NiAl-HT. The batch adsorption method was followed to study the sorption kinetics, and at different time intervals the concentration was monitored using UV-Vis spectrophotometer. Before and after adsorption, the catalyst's nature was monitored using powder XRD and it was observed that after the adsorption study the d-spacing (d(003)) increased (8.71 Å) compared with the parent NiAl-HT (7.48 Å). Further, the FT-IR spectra confirmed that after adsorption the intensity of the nitrate group was reduced due to the intercalation of dye into a layer of hydrotalcite material. The adsorption capacity of HT materials was tested at different pH values ranging from 4 to 10, and it was observed that the maximum adsorption capacity of 150 mg-dye/g-sorbent was achieved at pH 6. The authors concluded that the adsorption capacity is mainly due to the ion-exchange between the azo dye and nitrate ions in interlayer of NiAl-HT, and it was further explained based on the coulombic attraction between the dye molecule and NiAl-HT surface.
Pérez et al. synthesized nano-hydrotalcite/SiO2 composite and utilized it for the removal of Cr(VI) by the ion exchange mechanism.178 The composite materials were prepared using calcined SBA-15 by dispersing on NaOH solution followed by in situ treatment of Mg and Al nitrate solution to get HT formation on silica. The composite was filtered and calcined at 500 °C for 8 h before it was used. A chromium adsorption experiment was carried out by treating K2Cr2O7 solution with parent HT and nano HT/SiO2. The calcined HT retained a layered structure on exposure to aqueous chromium solution and this is called a ‘memory effect’, which was explained based on XRD experiments before and after the adsorption of Cr. The HT-SiO2 composite (98.6%) showed better adsorption capacity than pure HT (88.7%). The authors also observed that HT-SiO2 composite more efficiently adsorbs Cr(VI), and the data were found to fit the Freundlich isotherm.
HT possessing various functional groups in the inter-layers space revealed its potential as good adsorbents. Anions intercalated-HT materials act as anion scavengers for several common anions such as CO32−, C2O42−, SO42− and F− ions, oxy-anions and organic pollutants such as organic dyes, pesticide, and herbicide from aqueous medium. Further, HT helps to remove toxic effluents like heavy metal ions As, Th and U efficiently from the industrial waste to the environmentally acceptable level. The observed strong adsorption efficiency of the HT can be attributed to strong anion-exchange and adsorption capacity, mobility of the interlayer anions and also large surface areas HT material.
Silicate intercalation into hydrotalcite materials
Hydrotalcite (HT) materials offer an opportunity to introduce various anions into the interlayer. And attempts were made to introduce hard anions like silicate species into the layers of HT materials, where the intercalated silicate species act as pillaring, and are expected to create porosity, increase the thermal stability and bi-functional characteristics.36,179–181 The following section describes the different forms of silicate intercalated HT or composite materials and their application in various fields, especially in catalysis.The first report on polymerization of silicate anions into hydrotalcite materials was by Schutz et al., using silicic acid as the silica source.33 They reported silication of two different hydrotalcite materials, [Mg3Al(OH)8]+·Cl and [Al2Li(OH)8]+·Cl−. Microprobe analysis clearly showed that Cl− was replaced by silica on silicate intercalated materials. The solid state 27Al MAS NMR pattern of the intercalated materials showed only octahedral (Oh) coordination before and after the silication process on HT materials and found no Al–O–Si bond formation. A change in the XRD basal reflection was found after silication, and information derived from FT-IR spectroscopy on the intercalated silicate layer further showed a well resolved band at 1100–1000 cm−1. Similar results were observed for both the MgAl-HT and LiAl-HT materials.
Yun et al. took a new approach to prepare silicate intercalated LDH materials using TEOS (tetraethylorthosilicate) as the silicate source in a nitrogen atmosphere.34 The hexagonal shape of the HT-silicate crystal was obtained by TEM and strong vibration around 950–1200 cm−1 was observed on FT-IR. The silicon environment was followed by 29Si-MAS NMR, which indicates a different silicate orientation in HT-silicate materials. The developed materials were tested for disproportionation of 2-methyl 3-butyn-2-ol (MBOH). Overall, better conversion was observed in without HT-silicate materials, but bi-functional characteristics (acidic & basic) arose on silicate intercalated HT materials.
Del Arco et al. studied the effect of the Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio on the exchange of borate or silicate/HT materials from the HT-nitrate precursor using source (NH4)2B4O7·4H2O and Na2SiO3 respectively.182 The MAS NMR (27Al) showed a single resonance peak corresponding to Oh-coordination on all HT-materials. The authors observed three peaks centered around −92, −99 and −110 ppm on the HT-silicate material using 29Si MAS NMR, and these peaks were assigned to the Q2, Q3 and Q4 sites. Further, FT-IR spectra of HT-silicate material showed characteristic Si–O–Si peaks around 950–1200 cm−1, which described the silicate units in different polymerization states. The effect of calcination on the HT-silicate material was studied and it was found that there was formation of MgSiO3 crystalline material at 950 °C. The borate-HT material structure collapsed on calcination around 500 °C. Thermal analysis of the HT materials showed different weight loss values; silicate and borate HT-materials displayed less weight loss than nitrate-HT-material.
Al ratio on the exchange of borate or silicate/HT materials from the HT-nitrate precursor using source (NH4)2B4O7·4H2O and Na2SiO3 respectively.182 The MAS NMR (27Al) showed a single resonance peak corresponding to Oh-coordination on all HT-materials. The authors observed three peaks centered around −92, −99 and −110 ppm on the HT-silicate material using 29Si MAS NMR, and these peaks were assigned to the Q2, Q3 and Q4 sites. Further, FT-IR spectra of HT-silicate material showed characteristic Si–O–Si peaks around 950–1200 cm−1, which described the silicate units in different polymerization states. The effect of calcination on the HT-silicate material was studied and it was found that there was formation of MgSiO3 crystalline material at 950 °C. The borate-HT material structure collapsed on calcination around 500 °C. Thermal analysis of the HT materials showed different weight loss values; silicate and borate HT-materials displayed less weight loss than nitrate-HT-material.
Depége et al. studied the polymerization of silicate anions into HT by the two different methods of co-precipitation and ion-exchange.35 The silicate anions were intercalated into various HT materials (ZnAl–Cl, ZnCr–SO4) using sodium meta silicate at pH 9 and pH 12. The authors confirmed the presence of silicate species using FT-IR spectral studies, which showed a vibrational band around 1200 cm−1, typical of polymeric Si–O–Si species. The ion-exchange method showed greater expansion of the interlayer on silicate intercalated HT than with the co-precipitation method. Based on the 29Si MAS NMR studies, it was proposed that the silicon environment was a phyllosilicate type polymeric silicate species. The presence of aluminum in both Oh (+12.5 ppm) and Td (+54 ppm) co-ordination on silicate intercalated materials was evident from the 27Al-MAS NMR. The transfer of Al from Oh-sites of layer HT material to Td sites of silicate based zeolitic materials, which was evident from MAS NMR.
New NiMgAl-HT was synthesized with silicates in the interlayer anion using the co-precipitation method by Albertazzi et al.183 The properties of silicate intercalated HT material were compared with carbonate-containing HT materials. In the FT-IR spectra, the authors found a new strong band around 995 cm−1 characteristic of silicates in anti-symmetric Si–O vibrational mode. The XRD pattern of silicate intercalated HT was found with less crystalline and a slight shift of 2θ due to SiO4 units condensed in the same orientation in the interlayer of the HT materials. Further N2 sorption analysis showed a significant improvement in terms of the greater surface area and pore volume on silicate intercalated HT materials. Finally, the authors concluded that the incorporation of silicates into the lamellar HT materials modified the structural and textural properties.
Saber et al. prepared Pt supported on ZnAlSi-HT material by the wet impregnation method using chloroplatinic acid as the Pt source.184 Similar XRD patterns were observed for Pt supported and unsupported HT materials, as evidence that the Pt was supported on its external surface and not in the interlayer space. Also, XRD analysis showed no agglomeration of Pt on the surface of ZnAlSi-HT materials. The thermal analysis-DSC profile of Pt-supported material showed two exothermic peaks around 425 and 485 °C. This was due to the decomposition and oxidation of chloroplatinic acid at elevated temperatures. As dehydrogenation is one of the methods to generate hydrogen in situ, the Pt-supported HT materials were tested for the dehydrogenation of cyclohexane. In addition, the Pt-supported ZnAlSi-HT material was studied for cracking of n-hexane at different temperatures. Conversion on the dehydrogenation of cyclohexane increased with increasing temperature and reached a maximum of 38% at 450 °C. All the materials showed similar catalytic activity on n-hexane cracking and a maximum conversion of 84% at 350 °C was observed.
Yadav et al. carried out aldol condensation of benzaldehyde with heptanal using hexagonal mesoporous silica (HMS) added to calcined magnesium aluminum hydrotalcite (CHT).185 The aldol condensation reaction was studied in a batch reactor under a nitrogen atmosphere. Different loadings of HMS on HT were evaluated and a good yield was obtained from the sample having a high loading of 20% (w/w) CHT/HMS. The product jasminaldehyde selectivity of 86% was obtained with heptanal and a benzaldehyde molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 at 150 °C using 20% HMS/CHT. The results were explained on the basis of the bi-functional character of CHT/HMS, where the weak acid sites available on HMS helped the activation of benzaldehyde by protonation of the carbonyl group, subsequently which attack the enolate intermediated generated on basic sites.
5 at 150 °C using 20% HMS/CHT. The results were explained on the basis of the bi-functional character of CHT/HMS, where the weak acid sites available on HMS helped the activation of benzaldehyde by protonation of the carbonyl group, subsequently which attack the enolate intermediated generated on basic sites.
Very recently, Baskaran et al. prepared different concentrations of silicate anion intercalated MgAl-HT by an in situ sol–gel method and the materials were systematically characterized using XRD, FT-IR, N2 sorption, TGA-DSC, TEM and 29Si MAS NMR.36 The intercalated silicate anions were crystallized into porous species in the interlayer domains of the HT structure using tetrapropylammoniumhydroxide (TPAOH) solution. From the various analytical and spectroscopic studies, it was proposed that silicate anions were intercalated and coated on the surface of the layered hydrotalcite with the formation of solid solution of magnesium silicate-HT. Silicate anion intercalated MgAl-HT shows a higher surface area than pure HT.
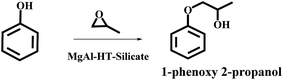
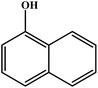
The silicate stabilized MgAl-HT was utilized for preparation of 1-phenoxy 2-propanol (Scheme 12) and the results are reproduced in Fig. 2. The authors found that silicate intercalated MgAl-HT (50%) yielded better conversion than pure MgAl-HT (7%); the observed higher conversion was explained based on the higher surface area and more exposed active species.
 | ||
| Fig. 2 Effect of catalyst on synthesis of 1-phenoxy 2-propanol using different HT materials (reproduced from ref. 36, with permission from the Royal Society of Chemistry). | ||
Zuo et al. prepared dual support of Ni containing MgAl-oxide and SBA-15 materials for reforming of methane.186 Three different kinds of materials were prepared; SBA-15 suspended in HT materials and subsequently calcined at 550 °C or 700 °C were labelled as SH-550 and SH-700. By contrast, the author prepared materials by direct mixing of SBA-15 and HT gel, representing these as MS-550 and MS-700, as well as 700 °C calcined HT materials suspended in SBA-15 followed by calcination at 550 °C, denoted as HS-550. In all cases, 10% NiO was introduced for the reforming process. From the TPR studies, the stability of the catalysts was found to be in the following order: HS-550 > SH-550 > MS-550 > SH-700 > MS-550. Further it was described from the TEM studies that the structure was maintained after reduction on HS-550 material. Ni particles were well dispersed on support materials. For the catalytic reforming process, HS-550 showed 85% conversion of CH4 and CO2 at 500 h. The higher activity was explained based on the strong metal (Ni)-support (SBA-15-HT) interaction. Among the various catalysts studied for the chosen reaction, the HS-550 (obtained from two calcinations) showed better catalytic stability and less carbon deposition.
In a continuation of previous work, Baskaran et al. studied alcohol oxidation using silicate intercalated CoAl-hydrotalcite (CoAl-HTSi) materials.187 The as-synthesized materials showed a layer structure, which is converted into a spinel matrix with uniform distribution on intercalated silica upon calcinations. The nature of the cobalt was analyzed using X-ray photoelectron spectroscopy (XPS). XPS showed two major peaks with satellite peaks, the major peaks corresponding to Co 2p3/2 and Co 2p1/2 with binding energy 782 and 797 eV respectively. From the XPS studies it was determined that Co was present in the mixed oxidation state (Co3+ and Co2+) with uniform distribution of Co3+ on CoAl-HTSi. The developed materials were tested for benzhydrol oxidation using TBHP in decane as the co-oxidant with the formation of benzophenone as the major product of 100%. The authors also monitored the reaction in the presence of radical scavenger BHT (butylated hydroxyl toluene) and found that there was a reduction in conversion. From the above result it was evident that the mechanism proceeded through a radical mechanism (Scheme 13). The catalyst also extended to various aliphatic and aromatic alcohols and the results are reproduced in Table 10.187 The presence of more exposed cobalt active species on CoAl-HTSi materials was responsible for the better catalytic activity.
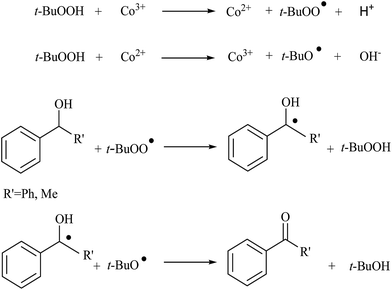 | ||
| Scheme 13 Plausible of mechanism of alcohol oxidation materials (reproduced from ref. 187 with permission from the Royal Society of Chemistry). | ||
| S. no. | Substrate | Catalyst | Product | Conv. (%) | Sel. (%) |
|---|---|---|---|---|---|
a Reaction conditions: alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxidant mole ratio of 1 oxidant mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; temperature = 70 °C; time = 6 h, CA-Si6-silicate intercalated CoAl-HT. 1; temperature = 70 °C; time = 6 h, CA-Si6-silicate intercalated CoAl-HT. |
|||||
| 1 | 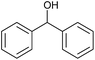 |
Without catalyst | 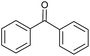 |
18.0 | 100 |
| 2 | 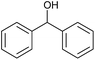 |
CA-Si6 | 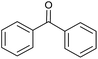 |
85.7 | 99.6 |
| 3 |  |
CA-Si6 | 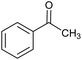 |
80.1 | 95.7 |
| 4 | 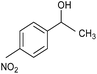 |
CA-Si6 | 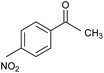 |
66.5 | 96.5 |
| 5 | 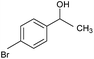 |
CA-Si6 | 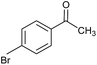 |
68.0 | 96.0 |
| 6 | 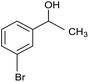 |
CA-Si6 | 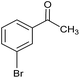 |
74.7 | 77.6 |
| 7 | 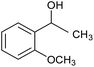 |
CA-Si6 | 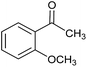 |
78.2 | 77.6 |
| 8 |  |
CA-Si6 | 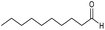 |
90.3 | 97.0 |
The same research group further developed a new composite material by introducing bulky SBA-15 mesoporous materials into MgAl-hydrotalcite (MASBA-HT).188 Both SBA-15 and HT nature were identified by the low and wide angle XRD of composite MASBA-HT. This was the first report of the presence of a layered HT structure on MASBA-HT even after calcinations at 550 °C, and the observed stability was explained based on the covalent bonding of silicates (from SBA-15) with a layered Mg and Al center through (Mg–O–Si and Si–O–Al) linkage. The above fact was supported by 27Al MAS NMR of calcined MASBA-HT composite, where it was found that there were two distinct peaks corresponding to Al3+ in Td (58 ppm) derived from the silicate framework and octahedral in the HT structure (7.6 ppm). The developed material's intrinsic properties were tested for hydro-isomerization of 1-octene in the vapor phase condition, as the hydro-isomerized products helped to improve the fuel efficiency. It was found that the composite materials (Fig. 3) showed a higher conversion of about 80% than the parent HT (55%) and SBA-15 (10%) materials at Weight Hour Space Velocity (WHSV) of 8 h−1. The presence of moderate acidity derived from Al in SBA-15 and basicity on HT together facilitate the higher catalytic activity on MASBA-HT composite.
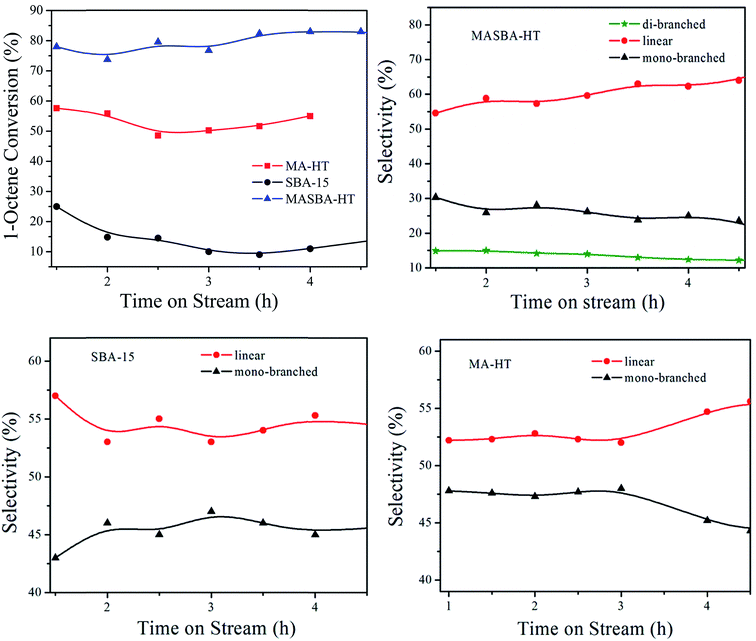 | ||
| Fig. 3 Effect of catalyst on hydro-isomerization of 1-octene and its selectivity at 450 °C, WHSV 8 h−1 (adapted from ref. 188 with kind permission from Elsevier). | ||
Another attempt was made to synthesize redox catalyst by mesoporous silica (SBA-15) intercalated CoAl-hydrotalcite materials (CoAl-HT-SBA).189 Different concentrations of SBA-15 containing gel were introduced into the pre-formed hydrotalcite gel and stirred for 24 h for effective intercalation. The developed redox catalysts were utilized for oxidation of ethylbenzene in liquid-phase medium using TBHP in decane as co-oxidant. Several kinetic parameter variations, such as oxidant, temperature, different catalysts and oxidant, substrate ratios, were evaluated and it was found that a higher ethylbenzene conversion of around 38% was obtained in optimum conditions (Table 11). In all these cases acetophenone was observed as the major product with a maximum selectivity of 72% and phenyl ethanol was formed as a minor product. The presence of surface-exposed cobalt active species was shown as responsible for the better catalytic activity on mesoporous CoAl-HT-SBA than parent CoAl-HT.
| S. no. | Catalyst | Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|---|
| Acetophenone | Phenyl ethanol | Benzoic acid | |||
a Reaction details: ethylbenzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBHP = 1 TBHP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, temperature: 100 °C, time: 6 h, catalyst: 100 mg, b, c-are 1st and 2nd recycle; d-2 mmol radical scavenger (BHT), e-physical mixture of calcined CA-HT and SBA-15. 1, temperature: 100 °C, time: 6 h, catalyst: 100 mg, b, c-are 1st and 2nd recycle; d-2 mmol radical scavenger (BHT), e-physical mixture of calcined CA-HT and SBA-15. |
|||||
| 1 | Without catalyst | 9.20 | 44.5 | 34.4 | 21.0 |
| 2 | SBA-15 | 6.30 | 34.1 | 65.8 | — |
| 3 | CoAl-HT | 24.2 | 60.4 | 26.1 | 13.3 |
| 4 | CoAl-HT-0.5SBA | 33.3 | 72.3 | 18.5 | 9.1 |
| 5 | CoAl-HT-1SBA | 38.4 | 72.0 | 19.1 | 8.8 |
| 6 | CoAl-HT-2SBA | 43.9 | 62.1 | 25.1 | 12.6 |
| 7b | CoAl-HT-1SBA | 39.5 | 64.4 | 26.1 | 9.4 |
| 8c | CoAl-HT-1SBA | 44.9 | 32.8 | 55.5 | 11.5 |
| 9d | CoAl-HT-1SBA | 7.20 | 27.4 | 48.6 | 23.8 |
| 10e | CA-HT/SBA-15 | 30.0 | 79.2 | 13.9 | 6.7 |
Verdejo et al. attempted to prepare nano-composite material using mixed oxides of Mg and Al (derived from MgAl-hydrotalcite on calcination) and mesoporous SBA-15 materials.190 This composite material was prepared by two different methods; (a) post-synthetic methods by adding MgAl-HT gel into either non-calcined or calcined SBA-15, (b) in situ synthetic method by introducing MgAl-HT gel into SBA-15 gel under stirring conditions. In all cases, the Mg and Al ratio was kept constant and the final materials were calcined at 550 °C before being utilized. An interesting result was observed on the textural properties of in situ synthesis, where the surface area and micropore area were found to be higher compared to other composite materials. From the SEM and TEM images of the composite materials the retaining pore channels of the mesoporous SBA-15 were evident. The developed materials were tested for water sorption at 60 °C at different ranges of relative humidity. The in situ prepared composite materials showed higher water sorption capacity (14.5 mmol of H2O/gcomposite) than the parent materials (5 mmol of H2O/gHT), a behaviour which was explained on the basis of pore diameter.
Baskaran et al. later utilized silicate intercalated HT both as a support to disperse cobalt nanoparticles and for hydroformylation reaction.191 The cobalt nanoparticles range of 1–15 wt% loaded in the SBA-15 intercalated MgAl-HT composites (MSB) support. Their HR-TEM analysis showed uniform spherical cobalt nanoparticles in the range of 3–10 nm. The retention of a layered structure even at a higher loading of about 15% Co on MSB materials was confirmed from their powder XRD study. The authors confirmed from FT-IR spectral studies that there is no spinel formation. The authors also found a decrease in the surface area and pore volume upon increase in loading of Co on MSB support, which was explained based on the blocking of pores by cobalt nanoparticles. The materials were tested for hydroformylation of olefin using syn-gas (CO/H2) under a liquid phase medium (Scheme 14). The maximum conversion of about 66% was observed on 5 wt% with exclusive formation of hydroformylated product (Table 12).
| Catalysts | Con. (%) | TOF (h−1) | Product selectivity (%) | Aldehyde distribution | ||
|---|---|---|---|---|---|---|
| H | CHO | Linear | Branched | |||
| a Reaction conditions: temperature = 200 °C; pressure = 80 bar, 1-octene: 0.0191 mole (3 mL), catalyst: 0.15 g, time = 8 h, H = hydrogenated product, CHO = hydroformylated products. | ||||||
| Without catalyst | 0.9 | — | 8.6 | — | — | — |
| MgAl-HT | 1.9 | — | 12.6 | — | — | — |
| SBA-15 | 2.0 | — | — | — | — | — |
| MSB | 3.5 | — | 2.2 | — | — | — |
| 1CoNMSB | 2.6 | 02.4 | 0.0 | 100.0 | 47.7 | 52.3 |
| 5CoNMSB | 65.7 | 12.4 | 0.0 | 100 | 44.3 | 55.7 |
| 12CoNMSB | 61.9 | 05.0 | 7.2 | 92.8 | 38.4 | 61.6 |
| 15CoNMSB | 54.1 | 01.8 | 0.0 | 100.0 | 35.0 | 65.0 |
Sakthivel et al. recently reported the first example of molybdenum carbonyl grafted on diaminosiloxane-functionalized cobalt aluminum-hydrotalcite (CA-HT) as a potential catalyst for the hydroformylation of 1-octene.192 The catalyst was prepared by a two-step procedure. First, silicate intercalated CoAl-hydrotalcite (CA-HTSi) support was functionalized using a calculated amount of N-[3-(trimethoxysilyl)propyl]ethylenediamine on the CA-HTSi materials, which the authors represented as CA-HTSi-DA. In the second step, Mo(CO)6 was grafted on CA-HTSi-DA materials under N2 atmosphere. Amine functionalization was confirmed with the help of 29Si MAS NMR, where it was found that peaks were present around −45 to −25 ppm corresponding to organosilica species. The authors confirmed the presence of carbonyl group in the materials by FT-IR, and the spectra of CA-HTSi-DA-Mo samples showed new vibrational bands around 2010, 1986 and 1930 cm−1, which are assigned to CO-moiety from Mo(CO)6. The prepared new material was tested for hydroformylation of 1-octene using syn-gas mixture (CO/H2) in liquid phase conditions. The authors tested the hydroformylation reaction using various catalysts and the results are reproduced in Table 13. The catalyst showed a maximum of 91% conversion with selective formation of branched aldehyde.
| Catalysts | Conv. (%) | Selectivity of productsb (%) | Distribution of aldehyde (%) | |||
|---|---|---|---|---|---|---|
| H | CHO | OH | Linear | Branched | ||
| a Reaction conditions: temperature = 200 °C; pressure = 90 bar, 1-octene: 0.0191 mole (3 mL), catalyst: 0.15 g, time = 8 h.b H = hydrogenated product, CHO = hydroformylated products, OH = alcohol products. | ||||||
| CA-HTSi | 40.0 | 5.7 | 32 | 0.0 | 33.6 | 67.4 |
| CA-HTSi-DA | 0.5 | 0.5 | 0.0 | 0.0 | 00.0 | 00.0 |
| CA-HTSi-DA-1Mo | 51.0 | 20 | 9.7 | 21.3 | 03.5 | 96.5 |
| CA-HTSi-DA-2Mo | 82.0 | 2.3 | 71 | 8.7 | 22.5 | 77.5 |
| CA-HTSi-DA-3Mo | 87.0 | 0.0 | 85 | 7.5 | 20.3 | 79.7 |
| CA-HTSi-DA-4Mo | 91.0 | 0.8 | 81 | 9.6 | 20.5 | 79.5 |
The amine functionalized silicate-anion-intercalated hydrotalcite (MA-HTSi-DA) materials were also a catalyst for nitroaldol condensation with nitromethane (Scheme 15).193 The catalyst was prepared by grafting diaminosilane (N-[3-(trimethoxysilyl)propyl]ethylenediamine) onto the surface using the post-synthesis method on MA-HTSi. The authors confirmed the amine functionality using FT-IR spectra by the presence of new vibrational bands around 2950–2740, 1480 and 1570 cm−1, corresponding to –CH stretching, bending and –NH group in diaminosilane moiety respectively. The surface area and pore volume were found to be reduced on the amine functionalized HT compared with the parent HT materials. Further, functionalization was confirmed by 29Si MAS NMR, which showed a peak around −53 ppm typical of an organosilica environment (T site). The authors used the different functionalized HTSi materials for nitroaldol condensation and the results are reproduced in Table 14. The MgAl-HTSi-DA material demonstrated better activity for nitroaldol condensation, with 96% conversion and 99.5% dehydrated product (1).
 | ||
| Scheme 15 Nitroaldol condensation of benzaldehyde and nitromethane using diaminesilane functionalized HTSi-materials. | ||
| S. no. | Catalyst | Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|
| 1 | 2 | |||
a Reaction conditions: benzaldehyde (5 mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitromethane (15 mmol), catalyst: 100 mg, temperature: 70 °C, time: 2 h.b Temperature: 90 °C, time: 2 h; 1,2,3 = (N-[3-(trimethoxysilyl)propyl]ethylenediamine) functionalized MgAl-HT, CoAl-HT and ZnAl-HT respectively; 4-(3-anilinopropyl)tri-methoxysilane functionalized MgAl-HT 5-(3-aminopropyl)tri-ethoxysilane functionalized MgAl-HT. nitromethane (15 mmol), catalyst: 100 mg, temperature: 70 °C, time: 2 h.b Temperature: 90 °C, time: 2 h; 1,2,3 = (N-[3-(trimethoxysilyl)propyl]ethylenediamine) functionalized MgAl-HT, CoAl-HT and ZnAl-HT respectively; 4-(3-anilinopropyl)tri-methoxysilane functionalized MgAl-HT 5-(3-aminopropyl)tri-ethoxysilane functionalized MgAl-HT. |
||||
| 1 | MA-HTSi-DA4 | 96 | 99.5 | 0.5 |
| 2 | CA-HTSi-DA4 | 70 | 97.0 | 3.0 |
| 3 | ZA-HTSi-DA4 | 36 | 98.0 | 2.0 |
| 4 | MA-HTSi-PAn4 | 45 | 80.5 | 19.5 |
| 5 | MA-HTSi-PA4b | 61 | 100 | — |
The above section describe the importance on introduction of hard anions like silicate into the interlayer space of HT to generate more exposed surface active sites, surface area, pore volume and also help to get multi-functionality. The derived multi-functionality and porosity facilitate enhanced activity for many catalytic processes. The introduction of silicate anion into inter-layered HT materials produce pillared clay type materials, which found to be potential support for dispersion of nanoparticles. Further, intercalation enhances thermal stability of silicate anion intercalated HT which helps to utilize HT for reactions involving vapor phase conditions.
Conclusion
The review provides insights into nature of active sites of hydrotalcite by tuning the various framework metal ions and interlayer anions which generate efficient catalyst and catalytic support etc. In particular, the homogeneously distributed mixed oxides obtained using various divalent and trivalent metal ions are shown as promising catalysts for variety of organic transformations like aromatic alkylation, C–C coupling, condensation, base catalysed addition, esterification and redox reactions. Intercalation of various anions results in new avenues for heterogeneous catalysis. Ability to incorporate transition metal ions into the framework octahedral position facilitates utilization of HT materials for various oxidation and reduction processes. Further, these materials have high flexibility to tune the active sites through either framework cations or interlayer anions, which facilitate introduction of multi-functionality. In recent years, much research work has been focused on the stabilization of interlayer space by introducing hard anions viz., silicates, which have opened up as a new class of porous composite material possessing a high surface area and uniformly distributed active sites which improve the catalytic activity. In particular, the silicate intercalated and stabilized magnesium-aluminium hydrotalcite stabilize its layered structure more than 400 °C and showed promising activity for various industrially important catalytic reactions such as hydroisomerization and aromatic alkylation. The scope for improving structural stability is the subject of further investigation.Acknowledgements
Authors extend his sincere and grateful to UGC (no. 41-237/2012/(SR)), DST and DU R&D (DRCH/R&D/2015-16)/RC/2015/9677/882 University of Delhi, New Delhi for the financial support. The authors also wish to express sincere thanks to USIC, Delhi University for instrumentation facility.References
- H. H. Murray, Appl. Clay Sci., 1991, 5, 379 CrossRef CAS.
- F. Cavani, F. Trifiró and A. Vaccari, Catal. Today, 1991, 11, 173 CrossRef CAS.
- A. Vaccari, Catal. Today, 1998, 41, 53 CrossRef CAS.
- J.-H. Choy, S.-J. Choi, J.-M. Oh and T. Park, Appl. Clay Sci., 2007, 36, 122 CrossRef CAS.
- A. I. Khan and D. O'Hare, J. Mater. Chem., 2002, 12, 3191 RSC.
- Layered Double Hydroxides-Structure and Bonding, ed. X. Duan and D. G. Evans, Springer-Verlag Berlin, Germany, 2005, p. 119 Search PubMed.
- S. Carlino, Solid State Ionics, 1997, 98, 73 CrossRef CAS.
- V. Rives and M. A. Ulibarri, Coord. Chem. Rev., 1999, 181, 61 CrossRef CAS.
- S. J. Mills, A. G. Christy, J. M. R. Genin, T. Kameda and F. Colombo, Mineral. Mag., 2012, 76, 1289 CrossRef CAS.
- S. Nishimura, A. Takagaki and K. Ebitani, Green Chem., 2013, 15, 2026 RSC.
- G. Fan, F. Li, D. G. Evans and X. Duan, Chem. Soc. Rev., 2014, 43, 7040 RSC.
- J. Feng, Y. He, Y. Liu, Y. Du and D. Li, Chem. Soc. Rev., 2015, 44, 5291 RSC.
- K. Goh, T. Lim and Z. Dong, Water Res., 2008, 42, 1343 CrossRef CAS PubMed.
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124 CrossRef CAS PubMed.
- M. B. Gawande, R. K. Pandeyb and R. V. Jayaram, Catal. Sci. Technol., 2012, 2, 1113 CAS.
- S. Kannan, Catal. Surv. Asia, 2006, 10, 117 CrossRef CAS.
- B. F. Sels, D. E. de Vos and P. A. Jacobs, Catal. Rev.: Sci. Eng., 2001, 43, 443 CAS.
- D. Tonelli, E. Scavetta and M. Giorgetti, Anal. Bioanal. Chem., 2013, 405, 603 CrossRef CAS PubMed.
- T. Stimpfling, F. Leroux and H. H. Bruening, Eur. J. Inorg. Chem., 2012, 5396 CrossRef CAS.
- C. Del Hoyo, Appl. Clay Sci., 2007, 36, 103 CrossRef CAS.
- W. T. Reichle, Solid State Ionics, 1986, 22, 135 CrossRef CAS.
- V. R. L. Constantino and T. J. Pinnavaia, Inorg. Chem., 1995, 34, 883 CrossRef CAS.
- M. Rajamathi, G. Thomas and P. V. Kamath, J. Chem. Sci., 2001, 113, 671 CrossRef CAS.
- W. Feitknecht, Helv. Chim. Acta, 1942, 25, 131 CrossRef CAS.
- G. Brown and M. C. Gastuche, Clay Miner., 1967, 7, 177 Search PubMed.
- O. Marino and G. Mascolo, Thermochim. Acta, 1982, 55, 377 CrossRef CAS.
- S. Miyata and T. Kumara, Chem. Lett., 1973, 843 CrossRef CAS.
- S. Miyata, Clays Clay Miner., 1975, 23, 369 CAS.
- S. Miyata and A. Okada, Clays Clay Miner., 1977, 25, 14 Search PubMed.
- F. Malherbe, L. Bigey, C. Forano, A. de Roy and J. P. Besse, J. Chem. Soc., Dalton Trans., 1999, 3831 RSC.
- V. R. L. Constantino and T. J. Pinnavaia, Inorg. Chem., 1995, 34, 883 CrossRef CAS.
- S. J. Palmer, A. Soisonard and R. L. Frost, J. Colloid Interface Sci., 2009, 329, 404 CrossRef CAS PubMed.
- A. Schutz and P. Biloen, J. Solid State Chem., 1987, 68, 360 CrossRef CAS.
- S. K. Yun, V. R. L. Constanting and T. J. Pinnavaia, Clays Clay Miner., 1995, 43, 503 CAS.
- C. Depége, F.-Z. EI Metoui, C. Forano, A. de Roy, J. Dupuis and J. P. Besse, Chem. Mater., 1996, 8, 952 CrossRef.
- T. Baskaran, R. Kumaravel, J. Christopher and A. Sakthivel, RSC Adv., 2013, 3, 16392 RSC.
- R. Rojas, C. Barriga, M. A. Ulibarri and V. Rives, J. Solid State Chem., 2004, 177, 3392 CrossRef CAS.
- J. Twu and P. K. Dutta, J. Catal., 1990, 124, 503 CrossRef CAS.
- V. Murthy, H. D. Smith, H. Zhang and S. C. Smith, J. Phys. Chem. A, 2011, 115, 13673 CrossRef CAS PubMed.
- M. A. Drezdzon, Inorg. Chem., 1988, 27, 4628 CrossRef CAS.
- E. D. Dimotakis and T. J. Pinnavaia, Inorg. Chem., 1990, 29, 2393 CrossRef CAS.
- U. Costantino, M. Casciola, L. Massinelli, M. Nocchetti and R. Vivani, Solid State Ionics, 1997, 97, 203 CrossRef CAS.
- M. Badreddine, A. Legrouri, A. Barroug, A. de Roy and J. P. Besse, Mater. Lett., 1999, 38, 391 CrossRef CAS.
- A. N. Ay, B. Z. Karan, A. Temel and L. Mafra, Appl. Clay Sci., 2011, 51, 308 CrossRef CAS.
- L. S. Li, S. J. Ma, X. S. Liu, Y. Yue, J. B. Hui, R. Xu, Y. M. Bao and J. Rocha, Chem. Mater., 1996, 8, 204 CrossRef CAS.
- J. Twu and P. K. Dutta, Chem. Mater., 1992, 4, 398 CrossRef CAS.
- X. Yu, J. Wang, M. Zhang, P. Yang, L. Yang, D. Cao and J. Li, Solid State Sci., 2009, 11, 376 CrossRef CAS.
- J. C. Villegas, O. H. Giraldo, K. Laubernds and S. L. Suib, Inorg. Chem., 2003, 42, 5621 CrossRef CAS PubMed.
- B. M. Choudary, N. S. Chowdari, M. L. Kantam and K. V. Raghavan, J. Am. Chem. Soc., 2001, 123, 9220 CrossRef CAS PubMed.
- S. K. Yun and T. J. Pinnavaia, Inorg. Chem., 1996, 35, 6853 CrossRef CAS PubMed.
- S. Omwoma, W. Chen, R. Tsunashima and Y. F. Song, Coord. Chem. Rev., 2014, 258–259, 58 CrossRef CAS.
- J. Zhang, F. Zhang, L. Ren, D. G. Evans and X. Duan, Mater. Chem. Phys., 2004, 85, 207 CrossRef CAS.
- M. Meyn, K. Beneke and G. Lagaly, Inorg. Chem., 1990, 29, 5201 CrossRef CAS.
- M. del Arco, S. Gutiérrez, C. Martín, V. Rives and J. Rocha, J. Solid State Chem., 2004, 177, 3954 CrossRef CAS.
- N. T. Whilton, P. J. Vickers and S. Mann, J. Mater. Chem., 1997, 7, 1623 RSC.
- B. M. Choudary, M. L. Kantam, C. V. Reddy, K. Koteswara Rao and F. Figueras, Green Chem., 1999, 187 RSC.
- K. Zou, H. Zhang and X. Duan, Chem. Eng. Sci., 2007, 62, 2022 CrossRef CAS.
- S. Fleutot, B. Dieudonne, J. C. Dupin, G. Renaudin and H. Martinez, Thermochim. Acta, 2012, 538, 1 CrossRef CAS.
- L. Latterini, M. Nocchetti, G. G. Aloisi, U. Costantino, F. C. de Schryver and F. Elisei, Langmuir, 2007, 23, 12337 CrossRef CAS PubMed.
- F. R. Costa, A. Leuteritz, U. Wagenknecht, D. Jehnichen, L. Haubler and G. Heinrich, Appl. Clay Sci., 2008, 38, 153 CrossRef CAS.
- V. Prevot, C. Forano and J. P. Besse, Appl. Clay Sci., 2001, 18, 3 CrossRef CAS.
- Y. Kohno, K. Totsuka, S. Ikoma, K. Yoda, M. Shibata, R. Matsushima, Y. Tomita, Y. Maeda and K. Kobayashi, J. Colloid Interface Sci., 2009, 337, 117 CrossRef CAS PubMed.
- I. Carpani, M. Berrettoni, M. Giorgetti and D. Tonelli, J. Phys. Chem. B, 2006, 110, 7265 CrossRef CAS PubMed.
- Z. Huang, P. Wu, X. Zhang, X. Wang, N. Zhu, J. Wu and P. Li, Appl. Clay Sci., 2012, 65–66, 87 CrossRef CAS.
- E. Coronado, C. M. Gastaldo, E. N. Moratalla and A. Ribera, Appl. Clay Sci., 2010, 48, 228 CrossRef CAS.
- M. del Arco, S. Gutiérrez, C. Martín and V. Rives, Inorg. Chem., 2003, 42, 4232 CrossRef CAS PubMed.
- E. L. Salinas and Y. Ono, Microporous Mater., 1993, 1, 33 CrossRef.
- K. Lang, P. Bezdička, J. L. Bourdelande, J. Hernando, I. Jirka, E. Káfuňková, F. Kovanda, P. Kubát, J. Mosinger and D. M. Wagnerová, Chem. Mater., 2007, 19, 3822 CrossRef CAS.
- S. Bhattacharjee and J. A. Anderson, Adv. Synth. Catal., 2006, 348, 151 CrossRef CAS.
- S. Hamada, K. IKeue and M. Machida, Chem. Mater., 2005, 17, 4873 CrossRef CAS.
- E. M. Sabbar, M. E. de Roy and F. Leroux, Microporous Mesoporous Mater., 2007, 103, 134 CrossRef CAS.
- S. K. Sharma, V. K. Srivastava, R. S. Shukla, P. A. Parikhb and R. V. Jasra, New J. Chem., 2007, 31, 277 RSC.
- S. Singha, M. Sahoo and K. M. Parida, Dalton Trans., 2011, 40, 11838 RSC.
- A. Corma, F. Rey, J. M. Thomas, G. Sankar, G. N. Greaves, A. Cervilla, E. Liopisd and A. Ribeirad, Chem. Commun., 1996, 1613 RSC.
- A. C. Gomes, S. M. Bruno, C. A. Gamelas, A. A. Valente, M. Abrantes, I. S. Gonçalves, C. C. Romão and M. Pillinger, Dalton Trans., 2013, 42, 8231 RSC.
- F. Leroux and J. P. Besse, Chem. Mater., 2001, 13, 3507 CrossRef CAS.
- M. Meyn, K. Beneke and G. Lagaly, Inorg. Chem., 1990, 29, 5201 CrossRef CAS.
- F. Millange, R. I. Walton, L. Lei and D. O'Hare, Chem. Mater., 2000, 12, 1990 CrossRef CAS.
- S. Miyata and T. Kumura, Chem. Lett., 1973, 843 CrossRef CAS.
- F. M. Vichi and O. L. Alves, J. Mater. Chem., 1997, 7, 1631 RSC.
- K. A. Carrado, A. Kostapapas and S. L. Suib, Solid State Ionics, 1988, 26, 77 CrossRef CAS.
- V. Prevot, B. Casal and E. Ruiz-Hitzky, J. Mater. Chem., 2001, 11, 554 RSC.
- T. Challier and R. C. T. Slade, J. Mater. Chem., 1994, 4, 367 RSC.
- V. Prevot, C. Forano and J. P. Besse, Appl. Clay Sci., 2001, 18, 3 CrossRef CAS.
- H. Roussel, V. Briois, E. Elkaim, A. de Roy and J. P. Besse, J. Phys. Chem. B, 2000, 104, 5915 CrossRef CAS.
- A. M. Fogg, J. S. Dunn and D. O'Hare, Chem. Mater., 1998, 10, 356 CrossRef CAS.
- X. Hou and R. J. Kirkpatrick, Inorg. Chem., 2001, 40, 6397 CrossRef CAS PubMed.
- T. Stumpf, H. Curtius, C. Walther, K. Dardenne, K. Ufer and T. Fanghanel, Environ. Sci. Technol., 2007, 41, 3186 CrossRef CAS PubMed.
- T. Ikeda, H. Amoh and T. Yasunaga, J. Am. Chem. Soc., 1984, 106, 5772 CrossRef CAS.
- J. Inacio, C. Taviot-Gueho, S. Morlat-Therias, M. E. de Roy and J. P. Besse, J. Mater. Chem., 2001, 11, 640 RSC.
- E. Kanezaki, K. Kinugawa and Y. Ishikawa, Chem. Phys. Lett., 1994, 226, 325 CrossRef CAS.
- A. S. Prakash, P. V. Kamath and M. S. Hegde, Mater. Res. Bull., 2001, 35, 2189 CrossRef.
- L. Raki, D. G. Rancourt and C. Detellier, Chem. Mater., 1995, 7, 221 CrossRef CAS.
- W. Kagunya, Z. Hassan and W. Jones, Inorg. Chem., 1996, 35, 5970 CrossRef CAS.
- F. M. Labajos, M. J. Sanchez-Montero, M. J. Holgado and V. Rives, Thermochim. Acta, 2001, 370, 99 CrossRef CAS.
- F. Malherbe, C. Forano and J. P. Besse, J. Mater. Sci. Lett., 1999, 18, 1217 CrossRef CAS.
- J. M. Fernandez, C. Barriga, M.-A. Ulibarri, F. M. Labajos and V. Rives, J. Mater. Chem., 1994, 4, 1117 RSC.
- G. A. Caravaggio, C. Detellier and Z. Wronski, J. Mater. Chem., 2001, 11, 912 RSC.
- X. Wang, C. Yan, A. Sumboja, J. Yan and P. S. Lee, Adv. Energy Mater., 2014, 4, 1301240 Search PubMed.
- F. M. Labajos, M. D. Sastre, R. Trujillano and V. Rives, J. Mater. Chem., 1999, 9, 1033 RSC.
- U. Costantino, N. Coletti, M. Nocchetti, G. G. Aloisi and F. Elisei, Langmuir, 1999, 15, 4454 CrossRef CAS.
- Y. Israeli, C. T. Gueho, J. P. Besse, J. P. Morel and N. M. Desrosiers, J. Chem. Soc., Dalton Trans., 2000, 791 RSC.
- H. P. Boehm, J. Steinle and C. Vieweger, Angew. Chem., 1977, 89, 259 CrossRef CAS.
- R. Ma, K. Takada, K. Fukuda, N. Iyi, Y. Bando and T. Sasaki, Angew. Chem., Int. Ed., 2008, 47, 86 CrossRef CAS PubMed.
- P. Vialat, P. Rabu, C. Mousty and F. Leroux, J. Power Sources, 2015, 293, 1 CrossRef CAS.
- F. Gu, X. Cheng, S. Wang, X. Wang and P. S. Lee, Small, 2015, 11, 2044 CrossRef CAS PubMed.
- H. Fan, J. Zhu, J. Sun, S. Zhang and S. Ai, Chem.–Eur. J., 2013, 19, 2523 CrossRef CAS PubMed.
- R. Ma, Z. Liu, K. Takada, N. Iyi, Y. Bando and T. Sasaki, J. Am. Chem. Soc., 2007, 129, 5257 CrossRef CAS PubMed.
- M. del Arco, P. Malet, R. Trujillano and V. Rives, Chem. Mater., 1999, 11, 624 CrossRef CAS.
- X. Zou, A. Goswamy and T. Asefa, J. Am. Chem. Soc., 2013, 135, 17242 CrossRef CAS PubMed.
- Y. Li, L. Zhang, X. Xiang, D. Yan and F. Li, J. Mater. Chem. A, 2014, 2, 13250 CAS.
- P. Singh and R. Nagarajan, Mater. Lett., 2015, 159, 58 CrossRef CAS.
- J. P. Ramirez, S. Abello and N. M. van der Pers, Chem.–Eur. J., 2007, 13, 870 CrossRef PubMed.
- D. P. Debecker, E. M. Gaigneaux and G. Busca, Chem.–Eur. J., 2009, 15, 3920 CrossRef CAS PubMed.
- M. Shiraga, T. Kawabata, D. Li, T. Shishido, K. Komaguchi, T. Sano and K. Takehira, Appl. Clay Sci., 2006, 33, 247 CrossRef CAS.
- O. D. Pavel, R. Bîrjega, M. Che, G. Costentin, E. Angelescu and S. Serban, Catal. Commun., 2008, 9, 1974 CrossRef CAS.
- T. Selvam, A. Inayat and W. Schwieger, Dalton Trans., 2014, 43, 10365 RSC.
- M. R. Othman, Z. Helwani, Martunus and W. J. N. Fernando, Appl. Organomet. Chem., 2009, 23, 335 CrossRef CAS.
- D. Yan, Y. Zhao, M. Wei, R. Liang, J. Lu, D. G. Evans and X. Duan, RSC Adv., 2013, 3, 4303 RSC.
- B. Bi, L. Xu, B. Xu and X. Liu, Appl. Clay Sci., 2011, 54, 242 CrossRef CAS.
- T. Subramanian, A. Dhakshinamoorthy and K. Pitchumani, Tetrahedron Lett., 2013, 54, 7167 CrossRef CAS.
- M. A. Thyveetil, P. V. Coveney, H. C. Greenwell and J. L. Suter, J. Am. Chem. Soc., 2008, 130, 4742 CrossRef CAS PubMed.
- J. M. Oh, T. T. Biswicka and J. H. Choy, J. Mater. Chem., 2009, 19, 2553 RSC.
- M. J. Climent, A. Corma, S. Iborra, K. Epping and A. Velty, J. Catal., 2004, 225, 316 CrossRef CAS.
- P. K. Sahu, P. K. Sahu, S. K. Gupta and D. D. Agarwal, Catal. Sci. Technol., 2013, 3, 1520 CAS.
- P. M. Veiga, Z. S. B. Sousa, C. M. S. Polato, M. F. Portilho, C. O. Veloso and C. A. Henriques, J. Catal., 2013, 1–10, DOI:10.1155/2013/685063.
- R. Rahul, J. K. Satyarthi and D. Srinivas, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2011, 50, 1017 Search PubMed.
- S. J. Kim, Y. Lee, D. K. Lee, J. W. Lee and J. K. Kang, J. Mater. Chem. A, 2014, 2, 4136 CAS.
- P. Vialat, C. Mousty, C. T. Gueho, G. Renaudin, H. Martinez, J. C. Dupin, E. Elkaim and F. Leroux, Adv. Funct. Mater., 2014, 24, 4831 CrossRef CAS.
- S. Velu and C. S. Swamy, Catal. Lett., 1996, 40, 265 CrossRef CAS.
- B. M. Choudary, M. L. Kantam, C. V. Reddy, K. K. Rao and F. Figueras, J. Mol. Catal. A: Chem., 1999, 146, 279 CrossRef CAS.
- B. M. Choudary, B. Kavita, N. S. Chowdari, B. Sreedhar and M. L. Kantam, Catal. Lett., 2002, 78, 373 CrossRef CAS.
- A. H. Padmasri, A. Venugopal, V. D. Kumari, K. S. R. Rao and P. K. Rao, J. Mol. Catal. A: Chem., 2002, 188, 255 CrossRef CAS.
- V. R. Choudhary, D. K. Dumbre, P. N. Yadav and S. K. Bhargava, Catal. Commun., 2012, 29, 132 CrossRef CAS.
- M. J. Climent, A. Corma, S. Iborra and J. Primo, J. Catal., 1995, 151, 60 CrossRef CAS.
- D. Tichit, D. Lutic, B. Coq, R. Durand and R. Teissier, J. Catal., 2003, 219, 167 CrossRef CAS.
- S. K. Sharma, P. A. Parikh and R. V. Jasra, J. Mol. Catal. A: Chem., 2007, 278, 135 CrossRef CAS.
- L. Faba, E. Diaz and S. Ordonez, Appl. Catal., B, 2012, 113–114, 201 CrossRef CAS.
- D. Bharali, R. Devi, P. Bharali and R. C. Deka, New J. Chem., 2015, 39, 172 RSC.
- J. K. Mobley and M. Crocker, RSC Adv., 2015, 5, 65780 RSC.
- K. Zhu, C. Liu, X. Ye and Y. Wu, Appl. Catal., A, 1998, 168, 365 CrossRef CAS.
- B. M. Choudary, N. S. Chowdari, K. Jyothi and M. L. Kantam, J. Am. Chem. Soc., 2002, 124, 5341 CrossRef CAS PubMed.
- C. A. Antonyraj, M. Gandhi and S. Kannan, Ind. Eng. Chem. Res., 2010, 49, 6020 CrossRef CAS.
- H. B. Friedrich, M. Govender, X. Makhoba, T. D. Ngcobo and M. O. Onani, Chem. Commun., 2003, 2922 RSC.
- C. Cativiela, F. Figueras, J. M. Fraile, J. I. Garcia and J. A. Mayoral, Tetrahedron Lett., 1995, 36, 4125 CrossRef CAS.
- K. Kaneda, T. Yamashita, T. Matsushita and K. Ebitani, J. Org. Chem., 1998, 63, 1750 CrossRef CAS.
- T. Matsushita, K. Ebitani and K. Kaneda, Chem. Commun., 1999, 265 RSC.
- U. R. Pillai and E. S. Demessie, J. Mol. Catal. A: Chem., 2003, 191, 93 CrossRef CAS.
- I. Kirm, F. Medina, X. Rodríguez, Y. Cesteros, P. Salagre and J. Sueiras, Appl. Catal., A, 2004, 272, 175 CrossRef CAS.
- M. L. Kantam, R. Arundhathi, P. R. Likhar and D. Damodara, Adv. Synth. Catal., 2009, 351, 2633 CrossRef CAS.
- T. Mitsudome, A. Noujima, T. Mizugaki, K. Jitsukawa and K. Kaneda, Adv. Synth. Catal., 2009, 351, 1890 CrossRef CAS.
- D. Qiao, C. Xu and J. Xu, Catal. Commun., 2014, 45, 44 CrossRef CAS.
- W. Lv, L. Yang, B. Fan, Y. Zhao, Y. Chen, N. Lu and R. Li, Chem. Eng. J., 2015, 263, 309 CrossRef CAS.
- M. Dixit, M. Mishra, P. A. Joshi and D. O. Shah, J. Ind. Eng. Chem., 2013, 19, 458 CrossRef CAS.
- A. S. Nagpure, A. K. Venugopal, N. Lucas, M. Manikandan, R. Thirumalaiswamy and S. Chilukuri, Catal. Sci. Technol., 2015, 5, 1463 CAS.
- B. M. Choudary, M. L. Kantam, A. Rahman and C. V. Reddy, J. Mol. Catal. A: Chem., 2003, 206, 145 CrossRef CAS.
- Q. Shi, R. Lu, L. Lu, X. Fu and D. Zhao, Adv. Synth. Catal., 2007, 349, 1877 CrossRef CAS.
- P. Sangeetha, K. Shanthi, K. S. Rama Rao, B. Viswanathan and P. Selvam, Appl. Catal., A, 2009, 353, 160 CrossRef CAS.
- R. Jothiramalingam and M. K. Wang, Ind. Eng. Chem. Res., 2009, 48, 6162 CrossRef CAS.
- Y. C. Sharma, B. Singh and J. Korstad, Fuel, 2011, 90, 1309 CrossRef CAS.
- B. M. Choudary, M. L. Kantam, C. V. Reddy, S. Aranganathan, P. L. Santhi and F. Figueras, J. Mol. Catal. A: Chem., 2000, 159, 411 CrossRef CAS.
- H. Y. Zeng, Z. Feng, X. Deng and Y. Li, Fuel, 2008, 87, 3071 CrossRef CAS.
- S. H. Wang, Y. B. Wang, Y. M. Dai and J. M. Jehng, Appl. Catal., A, 2012, 439–440, 135 CrossRef CAS.
- G. D. Yadav and P. A. Chandan, Catal. Today, 2014, 237, 47 CrossRef CAS.
- H. R. Prakruthi, B. M. Chandrashekara, B. S. Jai Prakash and Y. S. Bhat, Catal. Sci. Technol., 2015, 5, 3667 CAS.
- S. Sankaranarayanan, G. Selvam and K. Srinivasan, RSC Adv., 2015, 5, 36075 RSC.
- D. Kishore and S. Kannan, J. Mol. Catal. A: Chem., 2004, 223, 225 CrossRef CAS.
- S. K. Sharma, V. K. Srivastava, R. S. Shukla, P. A. Parikh and R. V. Jasra, New J. Chem., 2007, 31, 277 RSC.
- I. Delidovich and R. Palkovits, J. Catal., 2015, 327, 1 CrossRef CAS.
- M. Jabłońska and R. Palkovits, Catal. Sci. Technol., 2015 10.1039/c5cy00646e.
- J. Inacio, C. T. Guého, C. Forano and J. P. Besse, Appl. Clay Sci., 2001, 18, 255 CrossRef CAS.
- J. Orthman, H. Y. Zhu and G. Q. Lu, Sep. Purif. Technol., 2003, 31, 53 CrossRef CAS.
- G. P. Gillman, Sci. Total Environ., 2006, 366, 926 CrossRef CAS PubMed.
- M. L. J. Núñez, M. T. Olguínaand and M. Solache Ríos, Sep. Sci. Technol., 2007, 42, 3623 CrossRef.
- A. Sampieri, G. Fetter, M. E. Castrejon, A. T. Cruz and P. Bosch, Beilstein J. Nanotechnol., 2011, 2, 99 CrossRef CAS PubMed.
- T. S. Anirudhan and S. Jalajamony, J. Environ. Sci., 2013, 25, 717 CrossRef CAS.
- M. N. Pahalagedara, M. Samaraweera, S. Dharmarathna, C.-H. Kuo, L. R. Pahalagedara, J. Gascon and S. L. Suib, J. Phys. Chem. C, 2014, 118, 17801 CAS.
- E. Pérez, L. Ayele, G. Getachew, G. Fetter, P. Bosch, A. Mayoral and I. Díaz, J. Environ. Chem. Eng., 2015, 3, 1555 CrossRef.
- M. Foulon and L. Stelandre, Patent, US 7,985,390 B2, 2011.
- P. Li, Y. Yu, P. P. Huang, H. Liu, C. Y. CaO and W. G. Song, J. Mater. Chem., 2014, 2, 339 RSC.
- Z. Gu, J. J. Atherton and Z. P. Xu, Chem. Commun., 2015, 51, 3024 RSC.
- M. del Arco, S. Gutiérrez, C. Martín, V. Rives and J. Rocha, J. Solid State Chem., 2000, 151, 272 CrossRef CAS.
- S. Albertazzi, F. Basile, P. Benito, P. Del Gallo, G. Fornasari, D. Gary, V. Rosetti and A. Vaccari, Catal. Today, 2007, 128, 258 CrossRef CAS.
- O. Saber, H. M. Gobara and A. A. Al Jaafari, Appl. Clay Sci., 2011, 53, 317 CrossRef CAS.
- G. D. Yadav and P. Aduri, J. Mol. Catal. A: Chem., 2012, 355, 142 CrossRef CAS.
- Z.-J. Zuo, C.-F. Shen, P.-J. Tan and W. Huang, Catal. Commun., 2013, 41, 132 CrossRef CAS.
- T. Baskaran, R. Kumaravel, J. Christopher and A. Sakthivel, RSC Adv., 2014, 4, 11188 RSC.
- T. Baskaran, J. Christopher, T. G. Ajithkumar and A. Sakthivel, Appl. Catal., A, 2014, 488, 119 CrossRef CAS.
- T. Baskaran, J. Christopher and A. Sakthivel, Adv. Porous Mater., 2014, 2, 54 CrossRef.
- A. P. Verdejo, A. Sampieri, H. Pfeiffer, M. R. Reyes, J.-D. Santamaría and G. Fetter, Beilstein J. Nanotechnol., 2014, 5, 1226 CrossRef PubMed.
- T. Baskaran, N. R. Mahato, J. Christopher and A. Sakthivel, Adv. Porous Mater., 2014, 2, 183 CrossRef.
- A. Sakthivel, N. R. Mahato, T. Baskaran and J. Christopher, Catal. Commun., 2015, 65, 55 CrossRef CAS.
- T. Baskaran, J. Christopher, S. Radhakrishnan and A. Sakthivel, J. Mol. Catal. A: Chem., 2015, 409, 11 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |