Electroluminescence and fluorescence response towards acid vapors depending on the structures of indole-fused phospholes†
Abstract
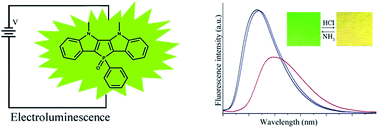
New isomers of phosphole heteroacenes 2-DIPO and 3-DIPO, in which indoles were fused with phospholes in different manners, have been synthesized. It is found that 2-DIPO containing the 5,6-dihydrophospholo[3,2-b:4,5-b′]diindole skeleton is highly emissive in solution and in solid state, but the emission of 3-DIPO bearing 5,7-dihydrophospholo[2,3-b:5,4-b′]diindole is weak because the emission of 2-DIPO and 3-DIPO is originated from π*–π and π*–n transitions, respectively. The OLED using 2-DIPO as emitter exhibits better performance than that based on 3-DIPO on account of the poor emission of 3-DIPO. It is interesting that the filter paper strip containing 2-DIPO could detect gaseous strong acid by naked eye. For example, the emitting color of the test paper changes from green to yellow upon exposure to HCl vapor, and can recover upon exposure to gaseous NH3. This is the first example of a fluorescence sensor probe based on phosphole to detect acid vapors. However, acid cannot induce the change of the emission for 3-DIPO due to the steric effect of neighboring groups of N–CH3 and benzene around P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to prevent the formation of H-bonding between acid and P
O to prevent the formation of H-bonding between acid and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in 3-DIPO.
O in 3-DIPO.

 Please wait while we load your content...
Please wait while we load your content...