Synthesis and highly efficient supercapacitor behavior of a novel poly pyrrole/ceramic oxide nanocomposite film
Hamid Mohammad Shiri*a,
Ali Ehsani*b and
Javad Shabani Shayehc
aDepartment of Chemistry, Payame Noor University, Iran. E-mail: hkmshiri@gmail.com
bDepartment of Chemistry, Faculty of Science, University of Qom, P. O. Box 37185-359, Qom, Iran. E-mail: ehsani46847@yahoo.com
cProtein Research center, University of Shahid Beheshti, Tehran, Iran
First published on 8th October 2015
Abstract
A novel electrochemical synthetic method for yttrium aluminum garnet (YAG:Al5Y3O12) was successfully developed in a mixture of YCl3 and AlCl3 aqueous solution. The electrosynthesized YAG was further annealed and characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM). A Ppy/YAG thin film electrode was synthesized electrochemically as an electrochemical supercapacitor. Scanning electron micrographs clearly reveal the formation of nanocomposites on the surface of the working electrode. Different electrochemical techniques, including galvanostatic charge–discharge (CD) experiments, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS), were used to investigate the applicability of the system as a supercapacitor. Based on the electrochemical results obtained, Ppy/YAG gave higher specific capacitance, power and energy values than Ppy at a current density of 1 mA cm−2. The specific capacitance (SC) of the Ppy and Ppy/YAG electrodes was calculated using the CV method, which was 109 and 254 F g−1, respectively. This study introduces new nanocomposite materials for electrochemical redox capacitors, which have advantages, such as long life cycle and stability in an aqueous electrolyte, to that of the commonly used ruthenium based perovskites.
1. Introduction
Electrochemical capacitors (ECs), which are known as supercapacitors, are charge-storage devices that are capable of very fast charge and discharge and have a unique combination of high power, high energy and long lifetime.1,2 ECs with these unique properties can bridge the gap between batteries and capacitors, thus offering great potential in applications such as starting automotives and regeneration of brake energy. To date, carbon,2–6 transition metal oxides4,5 and conducting polymers (CPs)6 have been identified as the most promising materials for ECs. Each material has its own unique advantages and disadvantages for supercapacitor applications.Some of the most famous CPs include polyaniline, polypyrrole, polythiophene and their derivatives. Polyaniline (PANI) and polypyrrole (Ppy) are the most promising CPs for application as electrodes in redox supercapacitors.7 The charge storage mechanism in CPs is followed by the loss of electrons and formation of polycations, which cause the anions in the solution to intercalate into the CP to maintain electroneutrality. The repeating charge–discharge process leads to the accumulation of stress on the polymer, which is related to poor cycle life.8 One of the solutions to this problem is the use of nanomaterials to synthesize nanocomposites. In these nanocomposites, nanoparticles are dispersed in a monomer solution to create an entangled polymer matrix.9,10
There are many ongoing attempts to increase the capacitance values of electrodes with a minimum investment. Because the existing electrode materials are highly expensive, alternative metal oxides are being explored. Most of the attempts have been made with a small amount of ruthenium, which serves as an excellent metal oxide for supercapacitors, with other materials for better performance. One of the attempts to obtain a better supercapacitor electrode performance was the use of a perovskite such as SrRuO3, which contained ruthenium.11,12 It is expected that combined metal oxides such as the perovskite BiFeO3 will show a similar or perhaps better performance and bismuth iron oxide in five crystallite phases, i.e. BiFeO3, Bi2Fe4O9 and Bi3Fe5O12 is well known for this, which means that this material may sustain charges in its phases during electrochemical changes.12
YAG has displayed numerous potential properties for optical and structural applications. Conventionally, YAG is prepared using solid-state combustion techniques. However, two intermediate phases, yttrium aluminum perovskite (YAP) and yttrium aluminum monoclinic (YAM), are produced during the heating period of this method. The hydrothermal method,13,14 in this case, with a pressure of over 40 MPa is required to form YAG. Although the sol–gel process seems to be a potential method,15 the precursory solution is so complicated that a high temperature (1050 °C) annealing process is essential for removing the impurity residues.16 Because luminescent materials for flat panel displays have attracted increasing attention, research activities aimed at developing these types of materials in the form of films are increasingly required.17 Therefore, the development of a simple process using a low cost equipment, at lower temperatures and at ambient atmosphere, is more attractive for commercialization. In this report, a YAG thin film was prepared by pulse electrochemical synthesis in a mixture of YCl3 and AlCl3 aqueous solution.
There are two methods for the preparation of conductive polymer/nanocomposites: the chemical method in which an oxidizing agent is used and in situ polymerization occurs18,19 and the other method is the electrochemical synthesis of conductive polymeric nanocomposites in which nanomaterials are dispersed in a monomer solution and a nanocomposite is formed by electropolymerization.20–26 Electrodeposition is a powerful and interesting process, which can be applied in numerous fields. Films can be synthesized at low or room temperature because of the high energy density accumulated in the solution near the electrode surface. The advantages of electrodeposition compared with other techniques include the low cost of raw materials and equipments, capability of controlling composition and morphology by varying electrochemical parameters and ability to deposit films on a complex surface. This is probably the easiest, non-vacuum and suitable method to prepare electrodes of large area. In the present study, a room temperature electrochemically synthesized Ppy/YAG electrode is presented as an efficient potential candidate for supercapacitor applications. Our goals in this study were to increase the capacitance of the Ppy electrode using YAG nanoparticles to form a composite electrode and then increase the cycle ability of the electrode.
2. Experimental
2.1. Reagent and materials
All the chemicals used in this study, obtained from Merck Chemical Co., were of analytical grade and were used without further purification. Double distilled water was used throughout the experiments. Pyrrole (py) was doubly distilled and the resulting colorless liquid was kept in the dark at 5 °C.2.2. Apparatus
All electrochemical experiments were carried out using an Autolab General Purpose System PGSTAT 30 (Eco-chime, Netherlands). A conventional three electrode cell with an Ag/AgCl reference electrode (Argental, 3 M KCl) was used to carry out the electropolymerization of Ppy. A platinum wire with a diameter of 0.5 mm and an exposed area of 0.65 cm2 was used as the counter electrode. A glassy carbon electrode with an area of 0.03 cm2 was used as the working electrode. A wide frequency range of 10 mHz to 100 kHz was used in EIS. Morphological investigations of the polymeric films were carried out using SEM (Philips XL 30). X-ray diffraction patterns were obtained on an X-ray diffractometer (PANalytical X'Pert-Pro) with a Cu-Kα monochromatized radiation source and an Ni filter.2.3. Preparation of YAG nanoparticles via cathodic pulse electrochemical deposition
YAG was electrosynthesized in a conventional three electrode cell with 316L stainless steel (100 × 50 × 0.5 mm) as the working electrode centered between two parallel graphite sheets (100 × 50 × 5 mm) as counter electrodes and saturated calomel electrode (SCE) as the reference electrode. The electrolyte was 0.005 M YCl3·6H2O and 0.008 M AlCl3·6H2O (with respect to YAG stoichiometry) dissolved in double-distilled water. The pH of the chemical bath was adjusted to 2.7 by adding a concentrated HCl solution. YAG thin films were deposited on the surface of stainless steel using the pulse voltage mode, with an applied peak current density of 5 mA cm−2 and ton = toff = 1 ms for 20 min at 60 °C. A square wave potential (−0.7 V with respect to the SCE) was applied in the cathodic electrodeposition of YAG. After the electrodeposition process, the steel electrodes were washed, dried at room temperature for 36 h and the deposited powders were scraped from the steel electrodes for further processing. Heat-treatment of the hydroxide powders was conducted at 1200 °C in dry air atmosphere for 2 h via an electrical furnace with a heating rate of 5 °C min−1. The transparent YAG powder was finally formed after annealing at 1200 °C. The YAG powder was characterized by means of X-ray diffraction, as shown in Fig. 1. In this figure, the strong and sharp peaks at 2θ = 18.19°, 29.74° and 33.42° are characteristic of YAG.SEM and EDX were applied for the analysis of the compound. The EDS spectrum and SEM image of YAG (Al5Y3O12) are shown in Fig. 2 and 3, respectively. As evident from the SEM image and EDS spectrum, the formation of YAG nanoparticles was confirmed.
2.4. Synthesis of Ppy and the Ppy/YAG nanocomposite
The Ppy/YAG nanocomposite was synthesized electrochemically by cyclic voltammetry in 0.1 M KCl solution containing Py monomer (0.1 M), YAG (0.5 wt%) and sodium dodecyl sulfate (0.005 M), which were dispersed in the solution by sonication. The Ppy electrode was synthesized in the same solution without YAG. Electropolymerization was conducted by 10 consecutive cycles at the sweep rate of 50 mV s−1 in potentials between 0.0 and 1 V. The mass of the Ppy films was approximated assuming a current efficiency for the electropolymerization process of 100%, using Faraday's law. The SEM images of the Ppy/YAG film are shown in Fig. 4. | ||
| Fig. 4 SEM images of the Ppy/YAG nanocomposite under different magnifications and quantitative results of the EDS analysis. | ||
3. Results and discussion
Fig. 5 shows the CVs of Ppy and the Ppy/YAG electrodes in 0.1 M H2SO4 solution at the sweep rate of 25 mV s−1. As observed, the capacitance of the composite electrode is about two times greater than that of the Ppy electrode, which shows that the use of YAG in the Ppy electrode enhances the capacity of the electrode. The synergetic effect that results from the interactions of Ppy and the YAG nanoparticles may affect the shape of the CV curves. The CV of the Ppy/YAG electrode exhibits almost symmetrical rectangular shapes in the potential window, which shows that the incorporation of YAG in the Ppy matrix not only increases the capacitance of the composite due to the redox transitions of YAG between different valence states, but also retains its ideal capacitive behavior. Due to electrostatic interactions, ions in the solution migrate to the electrode to counterbalance the charge on the electrode, i.e. the protons travel from one electrode to the other through the electrolyte during charge and discharge. The movement of electrons occurs at the same time through the current source or the external load. Thus, the current–voltage profile of the composite electrode is symmetric. | ||
| Fig. 5 Cyclic voltammograms of the Ppy and Ppy/YAG electrodes in 0.1 M H2SO4 at the sweep rate of 25 mV s−1. | ||
The specific capacitance (SC) of the electrodes was calculated from the CV curves using the following equation:27
 | (1) |
The SC of the Ppy and Ppy/YAG electrodes were found to be 109 and 254 F g−1, respectively. Park et al.28 obtained a specific capacitance for lead ruthenate pyrochlore up to 160 F g−1. Similarly, for lead ruthenate pyrochlore, Cao et al.29 and Bang et al.30 obtained the specific capacitance of 90 and 100 F g−1, respectively. These values are certainly better than those obtained from the perovskite, SrRuO3, which was co-precipitated at 800 °C (specific capacitance = 8 F g−1) by Mehrens et al.31
Fig. 6 shows the CV curves of the Ppy/YAG electrode at various scan rates in 0.1 M H2SO4 solution. As can be seen, the excellent capacitive performance of the Ppy/YAG electrode is also verified from these curves. According to the CV results, by increasing the scan rate, the current response of the composite film increases. This behavior can be related to the ideal capacitive behavior of the Ppy/YAG electrode. Furthermore, the good rectangular CV shape of the Ppy/YAG electrode remains constant at the scan rate of 100 mV s−1. The deviation from rectangularity of the CVs becomes obvious as scan rate increases. This phenomenon can be attributed to the electrolyte and film resistance, and this distortion is dependent on scan rate. By increasing the sweep rate, the active sites will not have enough time for reaction on the surface of the electrode.
 | ||
| Fig. 6 CVs of the Ppy/YAG electrode at different scan rates in 0.1 M H2SO4 in the potential window of −0.2–0.8 V. | ||
Fig. 7 shows the calculated specific capacitance of the Ppy/YAG electrode as a function of scan rate. As observed, the capacitance of the two electrodes decays over the entire range of scan rate because in fast sweep rates just the outer pores are used and the inner pores are not accessible for the doping/undoing process. As can be observed, at high scan rates the composite electrode loses its SC with a reduction pattern, which is the same as the Ppy electrode. This shows that Ppy/YAG does not block the pores of the polymer network. If these phenomena occur, the reduction of SC in the composite electrode is more than the Ppy electrode.
 | ||
| Fig. 7 Variation of the specific capacitance for the Ppy and Ppy/YAG electrodes as a function of scan rate in 0.1 M H2SO4 solution. | ||
The galvanostatic charge/discharge method was used to highlight the capacitance characteristic of the Ppy/YAG composite electrode. Fig. 8 shows the charge/discharge behavior of the Ppy and Ppy/YAG electrodes in the potential range from −0.2 to 0.8 V at a current density of 2.0 A g−1. As it can be observed, a triangular shape between this potential ranges is observed, which indicates the good columbic efficiency and ideal capacitive behavior of Ppy/YAG as an electrode for application in supercapacitors.
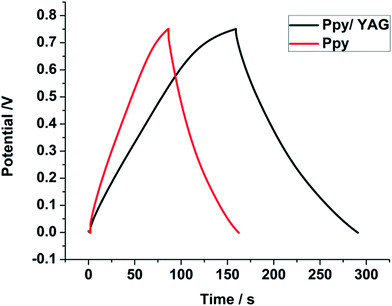 | ||
| Fig. 8 Galvanostatic charge and discharge measurements of the Ppy and Ppy/YAG electrodes in 0.1 M H2SO4 solution at the current density of 2.0 A g−1. | ||
Fig. 9 presents the charge–discharge curves of the Ppy/YAG electrode at various specific currents of 2.2–16 A g−1. As it can be observed, by enhancing the specific current, the specific capacitance values decrease due to the intercalation of ions at the surface of the active materials in the electrode/electrolyte interface. On the other hand, when a low specific current is used, the specific capacitance increases because there is enough time for insertion and deinsertion of the ions at the surface and inner pores of the active materials in the electrode/electrolyte interface. The phenomenon that can be concluded from this data is that in low density currents, the voltage range in the charge–discharge curves decrease. Herein, the SC was measured according to the charge/discharge curves, using eqn (2).
 | (2) |
 | ||
| Fig. 9 Galvanostatic charge–discharge curves of the Ppy/YAG electrode at 2.2–16 A g−1 in 0.1 M H2SO4 solution. | ||
The EIS technique was conducted to investigate the electrochemical supercapacitive and conductivity behaviors of the prepared Ppy/YAG electrode. Fig. 10 shows the Nyquist plots of the Ppy and Ppy/YAG electrodes at open circuit potential. The intercept of the Nyquist plots is related to the equivalent series resistance that arises from the contributions of electronic and ionic resistances.32–35 There is a semicircle at high frequencies, which is related to the charge transfer resistance (Rct) caused by the faradic reactions and the double-layer capacitance (Cdl) at the contact interface between the electrode and electrolyte solution. A resistance with the slope of the 45° in the curve, which is called the Warburg resistance (ZW), is a result of the frequency dependence of ion diffusion/transport in the electrolyte to the electrode surface. As observed, the magnitude of Rct in the Ppy/YAG electrode was smaller than that in the Ppy electrode, which shows that the addition of YAG improves the charge transfer performance of the Ppy composite electrode. In the low frequency region, both electrodes exhibit an almost linear branch, which indicates the decreased diffusion resistance of the electrolyte ions in the electrode, as expected for a capacitor. The low frequency capacitance (Clf) of each film was determined from the slope of the plot of the imaginary component of impedance at low frequency versus the inverse of frequency (f), using eqn (3).
 | (3) |
 | ||
| Fig. 10 Nyquist plots recorded from 10 kHz to 0.01 Hz with an ac amplitude of 5 mV for the Ppy and Ppy/YAG electrodes. | ||
It can be seen that the Ppy/YAG electrode has a higher capacitance than the Ppy electrode. The SC for the Ppy and Ppy/YAG electrodes was calculated to be 117 and 262 F g−1, respectively. These results also confirmed the data obtained by the CV and charge–discharge methods.
One of the most important parameters for practical application is cycling stability. As depicted in Fig. 11, the stability of the two electrodes is compared in terms of loss of their capacities as stability percentage. The stability of the electrode was calculated using the following equation:
 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, the Ppy/YAG electrode maintains its stability and retains more than 85% of its capacitance of the first cycle under consecutive cycles after 20
000 s, the Ppy/YAG electrode maintains its stability and retains more than 85% of its capacitance of the first cycle under consecutive cycles after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s.
000 s.
4. Conclusion
Polyaniline and polypyrrole are usually used as electrodes in redox SCs. However, due to the accumulation of stress on the polymer during the repeating charge–discharge process, the cycle life of pure CP-based SCs is poor, which needs to be further improved. For this purpose, the combination of conventional CP active materials and nanomaterials to fabricate hybrid electrodes has been considered to be one of the efficient approaches. In this study, first YAG was synthesized using the pulse electrochemical deposition technology and then hybrid Ppy/YAG films were fabricated by electro-polymerization of Ppy in the presence of YAG nanoparticles to serve as the active electrode for SCs. Compared with that of the pure Ppy electrode (109 F g−1), the obtained hybrid Ppy/YAG film electrode exhibits a much higher specific capacitance (254 F g−1) and excellent cycling stability. The enhanced capacitance performance may be attributed to the increase of conductivity and interfacial electron transfer dynamics by the incorporation of ceramic oxide nanoparticles, as evidenced by electrochemical impedance spectra. Furthermore, the Ppy/YAG composite electrode exhibited highly stable capacitance retention during charge/discharge cycling and is therefore a promising candidate for long-term applications in high-performance supercapacitors.Acknowledgements
The authors would like to express their deep gratitude to the Iranian Nano Council for supporting this study.References
- B. E. Conway, Electrochemical supercapacitors, Kluwer Academic, Plenum Publishers, New York, 1999 Search PubMed.
- J. Shabani Shayeh, P. Norouzi and M. R. Ganjali, RSC Adv., 2015, 5, 20446–20452 RSC.
- R. Kotz and M. Carlen, Electrochim. Acta, 2000, 45, 2483–2498 CrossRef CAS.
- C. Zhang, H. Yin, M. Han, Z. Dai, H. Pang, Y. Zheng, Y. Lan, J. Bao and J. Zhu, ACS Nano, 2014, 8(4), 3761–3770 CrossRef CAS PubMed.
- A. Zolfaghari, H. R. Naderi and H. R. Mortaheb, J. Electroanal. Chem., 2013, 697, 60–67 CrossRef CAS PubMed.
- A. Ehsani, M. Mahjani and M. Jafarian, Synth. Met., 2012, 162, 199–204 CrossRef CAS PubMed.
- K. S. Ryu, K. M. Kim, N.-G. Park, Y. J. Park and S. H. Chang, J. Power Sources, 2002, 103, 305–309 CrossRef CAS.
- M. E. Roberts, D. R. Wheeler, B. B. McKenzie and B. C. Bunker, J. Mater. Chem., 2009, 19, 6977–6979 RSC.
- Y. Chen, M. Han, Y. Tang, J. Bao, S. Li, Y. Lan and Z. Dai, Chem. Commun., 2015, 51, 12377–12380 RSC.
- A. Ehsani, M. G. Mahjani, F. Babaei and H. Mostaanzadeh, RSC Adv., 2015, 5, 30394–30404 RSC.
- M. W. Mehrens, J. Schenk, P. M. Wilde, E. Abdelmula, P. Axmann and J. Garche, J. Power Sources, 2002, 105, 182 CrossRef.
- C. D. Lokhande, T. P. Gujar, V. R. Shinde, R. S. Mane and S.-H. Han, Electrochem. Commun., 2007, 9, 1805–1809 CrossRef CAS PubMed.
- T. Takamori and L. D. David, Am. Ceram. Soc. Bull., 1986, 65, 1282 CAS.
- Y. Hakuta, T. Haganuma, K. Sue, T. Adschiri and K. Arai, Mater. Res. Bull., 2003, 38, 1257 CrossRef CAS.
- G. Gowda, J. Mater. Sci. Lett., 1986, 5, 1029 CrossRef CAS.
- S. Ramanathan, M. B. Kakade, S. K. Roy and K. K. Kutty, Ceram. Int., 2003, 29, 477 CrossRef CAS.
- Y. C. Wu, S. Parola, O. Morty, M. Villanutva-Ibaanez and J. Mugnier, Opt. Mater., 2005, 27, 1471 CrossRef CAS PubMed.
- M. A. Bavio, G. G. Acosta and T. Kessler, J. Power Sources, 2014, 245, 475–481 CrossRef CAS PubMed.
- Q. Xu, S.-X. Gu, L. Jin, Y.-e. Zhou, Z. Yang, W. Wang and X. Hu, Sens. Actuators, B, 2014, 190, 562–569 CrossRef CAS PubMed.
- A. Ehsani, A. Vaziri-Rad, F. Babaei and H. Mohammad Shiri, Electrochim. Acta, 2015, 159, 140–148 CrossRef CAS PubMed.
- A. Ehsani, M. Mahjani, M. Bordbar and S. Adeli, J. Electroanal. Chem., 2013, 710, 29–35 CrossRef CAS PubMed.
- A. Ehsani, M. Mahjani, M. Jafarian and A. Naeemy, Electrochim. Acta, 2012, 71, 128–133 CrossRef CAS PubMed.
- A. Ehsani, M. G. Mahjani, M. Jafarian and A. Naeemy, Prog. Org. Coat., 2010, 69, 510–516 CrossRef CAS PubMed.
- S. Naghdi, B. Jaleh and A. Ehsani, Bull. Chem. Soc. Jpn, 2015, 88, 722–728 CrossRef CAS.
- Z. Ežerskis and Z. Jusys, J. Appl. Electrochem., 2001, 31, 1117–1124 CrossRef.
- A. Ehsani, F. Babaei and H. Mostaanzadeh, J. Braz. Chem. Soc., 2015, 26, 331–337 CAS.
- J. Shabani-Shayeh, A. Ehsani, M. R. Ganjali, P. Norouzi and B. Jaleh, Appl. Surf. Sci., 2015, 353, 594–599 CrossRef CAS PubMed.
- B. O. Park, C. D. Lokhande, H. S. Park, K. D. Jung and O. S. Joo, Mater. Chem. Phys., 2004, 86, 239 CrossRef CAS PubMed.
- F. Cao and J. Prakash, J. Power Sources, 2001, 92, 40 CrossRef CAS.
- H. J. Bang, W. Lu, F. Cao and J. Prakash, Electrochem. Commun., 2000, 2, 653 CrossRef CAS.
- M. W. Mehrens, J. Schenk, P. M. Wilde, E. Abdelmula, P. Axmann and J. Garche, J. Power Sources, 2002, 105, 182 CrossRef.
- A. Pandolfo and A. Hollenkamp, J. Power Sources, 2006, 157, 11–27 CrossRef CAS PubMed.
- G. S. Gund, D. P. Dubal, S. S. Shinde and C. D. Lokhande, ACS Appl. Mater. Interfaces, 2014, 6, 3176–3188 CAS.
- A. Ehsani, M. G. Mahjani, R. Moshrefi, H. Mostaanzadeh and J. Shabani Shayeh, RSC Adv., 2014, 4, 20031–20037 RSC.
- Z. Fan, J. Yan, T. Wei, L. Zhi, G. Ning, T. Li and F. Wei, Adv. Funct. Mater., 2011, 21, 2366–2375 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |




