The critical role of additives in binary halogen-free solvent systems for the general processing of highly efficient organic solar cells†
Kai Yao*a,
Yun-Xiang Xub,
Xiaofeng Wangc,
Fan Lic and
Jiren Yuana
aInstitute of Photovoltaics, Nanchang University, 999 Xuefu Avenue, Nanchang 330031, China. E-mail: yaokai@ncu.edu.cn
bCollege of Polymer Science & Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, China
cDepartment of Materials Science and Engineering, Nanchang University, Nanchang 330031, China
First published on 20th October 2015
Abstract
With the rapid progress of organic solar cells (OSCs), avoiding processing with halogenated solvents has become an urgent task for the practical utilization of OSC technology. In this work, a non-halogenated solvent combination of methylbenzene and methylnaphthalene was developed to replace the use of the halogenated binary solvents chlorobenzene and 1-chloronaphthlene. We have systematically studied the effects of the binary solvents on the morphology, charge carrier mobility, and photovoltaic properties. Importantly, we have found that the halogen-free mixture solvent showed a wider applicability to various polymer:fullerene systems than that of the halogenated ones and realized a PCE of over 8% in the PTB7:PC71BM based OSC. Our work demonstrated that the use of the additive strategy for halogen-free solvent systems may provide a feasible route to address the critical environmental issues associated with large-scale manufacturing.
Introduction
Organic solar cells (OSCs) have the potential to reduce the costs and environmental impacts associated with other solar cells, while having the considerable advantages of being lightweight, flexible, and easily processed.1 As the power conversion efficiency (PCE) of solution processed OSCs based on bulk-heterojunction (BHJ) structures goes steadily over 10%,2 the development of OPV is approaching the requirement for practical application. The attention gained by organic photovoltaics will then be focused on fulfilling the commercial platforms necessary for deployment on a large-scale through roll-to-roll printing techniques.3 As is well known, conjugated polymers and fullerene derivatives have limited solubility in common solvents, and so halogenated solvents with a strong salvation effect,4 such as chlorobenzene (CB), 1,2-dichlorobenzene (o-DCB), diiodooctane (DIO), and 1-chloronaphthalene (1-CN), are used in solution processing. From the perspective of manufacturing, these halogenated solvents are generally not acceptable due to safety concerns and the need for environmentally-friendly processing conditions.5 Therefore, the search for non-halogenated solvents is urgently required in this transition toward future large-scale production.A BHJ layer consists of a phase-separated electron-donor (typically a conjugated polymer) and an electron-acceptor (typically a fullerene derivative) with ideal interpenetrating domains (∼10 nm in length), and the morphology of the BHJ layer has a huge influence on the charge generation, transfer, and transport.6 Such an ideal morphology can be difficult to obtain via simple solution processing because the thermodynamically metastable state is extremely sensitive to the solvent system used for processing.7 Thus, lots of physical properties of the solvents, including the boiling point (BP), toxicity, viscosity and solubility for the blended compounds, etc. need to be considered for processing.8 Hence, alternative non-halogenated solvents should have comparable physical properties to those of halogenated solvents, and a decent solubility for conjugated polymers and fullerene derivatives is essential to achieve high-performance OSCs.9
Compared to halogenated solvents, non-halogenated ones, such as toluene10 and isomers of xylene,11 usually lead to less-than-ideal morphologies in the BHJ layers due to their inferior physical properties for the drying process and solubility for conjugated polymers and fullerene derivatives. There are several reports that use binary solvent mixtures of differing compositions to provide one facile route to modulate the solvent quality. Even in halogenated solvents, the CB (DCB) host solvent has been widely used in many state-of-the-art photovoltaic OSC systems with 1-CN12 or DIO13,14 additives. In an attempt to rationally select candidate solvent mixtures, Hansen solubility parameters (HSP) have been utilized to describe the total cohesion energy, which is directly related to the solubility parameters (δ) (Table 1 and Fig. S1†).15 The application of solubility parameters has been utilized to understand the solubility of polymers and fullerenes in solvent mixtures. For example, Jen et al. used 1,2,4-trimethylbenzene/1,2-dimethylnaphthalene as a binary solvent to realize a PCE of over 7% in PIDTT-DFBT:PC71BM blends;16 Li et al. reported the use of toluene/N-methyl pyrrolidone (NMP) to make a P3HT:ICBA device.17 Recently, Hou et al. utilized the similar strategy and successfully demonstrated a high-performance PBDT-TS1/PC71BM solar cell with a PCE of over 9% by replacing DCB + DIO with o-xylene + NMP.18
| Solvents | Hansen solubility parameters (δD + δP + δH)a (MPa1/2) | Molar volumea (cm3 mol−1) | Boiling pointb (°C) | Densityb (g cm−3) | Vapor pressureb (kPa at 25 °C) | Workplace exposure limits (long-term)c |
|---|---|---|---|---|---|---|
| a From ref. 15.b “Laboratory Solvents and Other Liquid Reagents” in CRC Handbook of Chemistry and Physics, 90th edition (Internet Version 2010), David R. Lide, ed., CRC press/Taylor and Francis, Boca Raton, FL.c EH40/2005 Workplace exposure limits, second edition, 2011, Health and Safety Executive, Stationery Office publications, Norwich NR3 1GN. | ||||||
| Chlorobenzene | 19.0 + 4.3 + 2.0 | 102.1 | 131.7 | 1.11 | 1.6 | 1 ppm |
| Toluene | 18.0 + 1.4 + 2.0 | 106.8 | 110.6 | 0.89 | 3.79 | 50 ppm |
| o-Xylene | 17.8 + 1.0 + 3.1 | 121.2 | 144.5 | 0.88 | 0.88 | 50 ppm |
| 1-Chloronaphthalene | 19.9 + 4.9 + 2.5 | 136.2 | 259.3 | 1.19 | 0.004 | NA |
| 1,8-Diiodooctane | 17.6 + 4.8 + 4.6 | 199.2 | 332.5 | 1.84 | 0.0003 | NA |
| 1-Methylnaphthalene | 20.6 + 0.8 + 4.7 | 139.4 | 244.6 | 1.02 | 0.007 | NA |
| 1,2-Dimethylnaphthalene | 21.0 + 1.7 + 5.2 | 153.5 | 266.5 | 1.01 | 0.002 | NA |
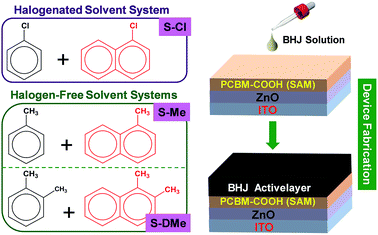
Considering that halogen-free solvents are still merely applied for a few polymers, it will be of great importance to the field if we can find a universal green solvent system to reproduce highly efficient OSCs which are based on current photovoltaic materials. Moreover, a matching principle (the choice of the additive solvent) in binary non-halogenated solvents is still missing. Here, we expand the matching role of a common halogenated solvent-system CB/CN (S-Cl)12 into halogen-free organic solvents, particularly toluene and o-xylene (Fig. 1). Specifically, the higher boiling point additive solvents 1-methylnaphthalene (MN) and 1,2-dimethylnaphthalene (DMN) are matched with toluene and o-xylene, from the principle of structural analogy (Scheme 1), to form a combination of S-Me and S-DMe. The halogen-free solvent methylnaphthalene was selected as the processing additive to optimize the less-than ideal morphologies. The initial device performance test revealed that the cells processed with non-halogenated binary solvents are comparable with those processed with the halogenated CB/CN solvent. Moreover, we find that our halogen-free solvents can also replace widely utilized solvents, such as the CB(DCB)–DIO mixture, in the fabrication of environmentally friendly devices, making this a class of general processing solvents for high performance OSCs.
 | ||
| Fig. 1 The matching role of non-halogenated and halogen-free solvents/additives (the system) for processing bulk heterojunction OSCs. | ||
Results and discussion
In this work, we chose the ladder-type polymer PIDT-FQ-T and the semi-crystalline conjugated polymer P3HT to test the device performance based on different solvent systems (Scheme 1). Most of the ladder-type polymers demonstrated an amorphous nature because their out-of-plane side chains inhibited their ordered interchain packing,19 while the devices based on P3HT were extremely sensitive to the morphology change.20 The polymer PIDT-FQ-T possesses a high charge carrier mobility and good solubility, and is quite insensitive to the processing conditions,21 while P3HT relies on post-casting treatments to enhance the crystallization of the polymer phase for better charge transport.22 Here, we chose the simple inverted device configuration of ITO/ZnO/PC61BM-COOH (SAM)/polymer:fullerene/MoOx/Ag (Fig. 1),23 in which fullerene-based PC61BM-COOH self-assembled monolayers (SAM) were used to modify the ZnO surface and facilitate the electron transport. Devices based on the PIDT-FQ-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
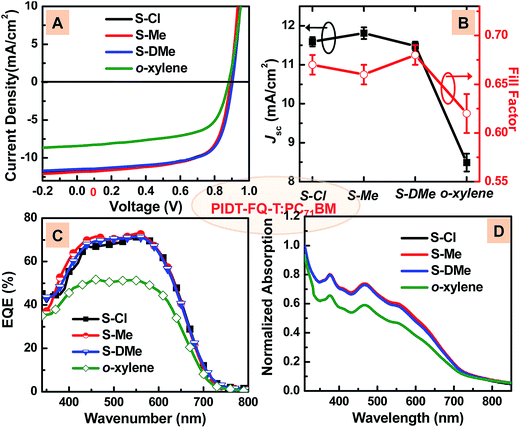
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, w/w) blend exhibited a PCE of 6.48% when the film was spin-coated from the CB solution, with an open-circuit voltage (VOC) = 0.90 V, a short-circuit current density (JSC) = 11.03 mA cm−2, and fill-factor (FF) = 0.65 (Fig. S2† and Table 2). The device performance can be further improved by adding CN to the solvent, for a better phase-separation morphology.12 CN, one of the popular halogenated additives, could help to create a finer phase separation in the active layer by improving the solubility of the conjugated polymers and increasing the drying time of the active materials, due to its low vapor pressure. As shown in Fig. 2A, the average PCE of the control device processed using CB + CN (2.5%) (S-Cl) is increased to 7.01%, with a JSC of 11.59 mA cm−2, VOC of 0.90 V and FF of 0.67. This result is consistent with the morphology variation. AFM topography images of the PIDT-FQ-T:PC71BM film cast from S-Cl (Fig. 3A) showed smaller domain sizes and a lower surface roughness than that of the pure CB solvent.
3, w/w) blend exhibited a PCE of 6.48% when the film was spin-coated from the CB solution, with an open-circuit voltage (VOC) = 0.90 V, a short-circuit current density (JSC) = 11.03 mA cm−2, and fill-factor (FF) = 0.65 (Fig. S2† and Table 2). The device performance can be further improved by adding CN to the solvent, for a better phase-separation morphology.12 CN, one of the popular halogenated additives, could help to create a finer phase separation in the active layer by improving the solubility of the conjugated polymers and increasing the drying time of the active materials, due to its low vapor pressure. As shown in Fig. 2A, the average PCE of the control device processed using CB + CN (2.5%) (S-Cl) is increased to 7.01%, with a JSC of 11.59 mA cm−2, VOC of 0.90 V and FF of 0.67. This result is consistent with the morphology variation. AFM topography images of the PIDT-FQ-T:PC71BM film cast from S-Cl (Fig. 3A) showed smaller domain sizes and a lower surface roughness than that of the pure CB solvent.
| Solventa | VOC (V) | JSC (mA cm−2) | FF | PCEb,c (%) | RMS (nm) |
|---|---|---|---|---|---|
| a The concentrations of additives are all 2.5% by volume.b The average device performance was obtained from 20 devices.c The maximum values of the PCEs are provided in parentheses. | |||||
PIDT-FQ-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 3) 3) |
|||||
| Chlorobenzene | 0.90 ± 0.01 | 11.03 ± 0.13 | 0.65 ± 0.01 | 6.48 ± 0.24 (6.93) | 0.94 |
| S-Cl | 0.90 ± 0.01 | 11.59 ± 0.12 | 0.67 ± 0.01 | 7.01 ± 0.21 (7.28) | 0.68 |
| S-Me | 0.89 ± 0.01 | 11.81 ± 0.15 | 0.66 ± 0.01 | 6.92 ± 0.23 (7.24) | 0.71 |
| S-DMe | 0.90 ± 0.01 | 11.48 ± 0.11 | 0.68 ± 0.01 | 7.18 ± 0.18 (7.39) | 0.62 |
| o-Xylene | 0.88 ± 0.02 | 8.49 ± 0.23 | 0.62 ± 0.02 | 4.62 ± 0.32 (5.16) | 1.81 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) IC60BA (1 IC60BA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1) |
|||||
| Chlorobenzene | 0.84 ± 0.01 | 10.13 ± 0.19 | 0.67 ± 0.02 | 5.65 ± 0.29 (6.05) | 1.42 |
| S-Cl | 0.84 ± 0.01 | 10.59 ± 0.14 | 0.68 ± 0.01 | 6.12 ± 0.21 (6.39) | 1.61 |
| S-Me | 0.83 ± 0.01 | 10.85 ± 0.16 | 0.69 ± 0.01 | 6.27 ± 0.25 (6.58) | 1.66 |
| S-DMe | 0.84 ± 0.01 | 10.92 ± 0.15 | 0.66 ± 0.01 | 6.02 ± 0.22 (6.35) | 1.54 |
| o-Xylene | 0.83 ± 0.02 | 8.31 ± 0.29 | 0.56 ± 0.02 | 3.85 ± 0.35 (4.31) | 4.46 |
 | ||
| Fig. 3 AFM tapping mode topography images (5 × 5 μm2) of the PIDT-FQ-T:PC71BM blend processed from (a) CB/CN (S-Cl), (b) toluene/MN (S-Me), (c) o-xylene/DMN (S-DMe) and (d) o-xylene. | ||
Then, we shifted our attention back to non-halogenated solvents and firstly investigated the photovoltaic characteristics of the PIDT-FQ-T:PC71BM devices processed from neat o-xylene solvents. The solar cells cast from o-xylene showed significantly inferior efficiencies compared to the over 6% efficiency of the CB solvent cast cells. The devices processed from o-xylene performed considerably better than the toluene-processed device, but only delivered PCEave = 4.62%, roughly half of that of the devices made from the S-Cl system. As we observed in Fig. 3, the PIDT-FQ-T:PC71BM film cast from the o-xylene solvent exhibited large clusters of a few hundred nanometers, while the film cast from halogenated solvents (CB or S-Cl) presented a homogeneous phase-separation with ideal morphology. The severe phase separation in the blend films processed with o-xylene would cause strong recombination, thereby leading to a very low photovoltaic performance.24 We assumed that the relatively low solubility of the conjugated polymers and fullerene in toluene or o-xylene could possibly be responsible for the severe phase separation.
In the preceding work we demonstrated that naphthalene-based additives have a more important function in non-halogenated solvents than in halogenated solvents, since they could significantly enhance the solubility and then boost the miscibility of the active-layer materials in the mixed solvents,10,25 according to the Hansen solubility parameters (Table 1). Moreover, from a structural view, the halogen-free methyl analogues of CN, MN and DMN are selected to match with toluene and o-xylene, respectively. And both of the film morphology based non-halogenated solvents are dramatically improved after addition of 2.5% naphthalene-based additives with a similar root mean square (RMS) roughness value. Although the film processing from halogenated CB/CN outperforms that from the o-xylene in the surface and bulk morphology, adding a small amount of 1, 2-dimethylnaphthalene to o-xylene could significantly meliorate the poor phase separation and film quality. The UV-vis spectra of the PIDT-FQ-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) films processed from different solvents are shown in Fig. 2D. We found that all of the S-Cl, S-Me and S-DMe cast films showed a similar light-harvesting behavior, and are significantly better than in the pure o-xylene case. These results imply that S-Me and S-DMe might perform as well as S-Cl in the processing of polymer:PCBM blends and act as good potential alternatives for high-performance OPV development.
3 w/w) films processed from different solvents are shown in Fig. 2D. We found that all of the S-Cl, S-Me and S-DMe cast films showed a similar light-harvesting behavior, and are significantly better than in the pure o-xylene case. These results imply that S-Me and S-DMe might perform as well as S-Cl in the processing of polymer:PCBM blends and act as good potential alternatives for high-performance OPV development.
Both devices fabricated from halogen-free co-solvents showed a more enhanced performance. The devices derived from S-Me showed average performance with VOC of 0.90 V, JSC of 11.81 mA cm−2, FF of 0.66, and overall PCE of 6.92%, which is close to that of the control device obtained from S-Cl. For the S-DMe system, a maximum PCE of 7.39% can be achieved, which is even a little better than that of the device fabricated by S-Cl. From the variations of the photovoltaic parameters, both of the two types of halogen-free co-solvents showed a high reproducibility for processing OSCs. As demonstrated in Fig. 2C, the external quantum efficiency (EQE) spectra of the devices processed by S-Cl, S-Me and S-DMe showed a similar trend to the J–V characteristics, much higher than that of the device processed by pure o-xylene. The combination of S-Cl, S-Me and S-DMe showed a comparable performance with similar photovoltaic parameters to the halogen system (Fig. 2B).
Both non-halogenated solvent fabricated devices showed a similar VOC around 0.9 V, similar to the CB-cast devices. However, for the pure o-xylene, we could observe decreases in both photocurrent and fill-factor. The increased series resistance (Rs) from the slopes of the experimentally obtained J–V curves implied poor charge transport and strong recombination of excitons through the polymer:fullerene composite. This result was in good agreement with the dependence of VOC on the light intensity, as shown in Fig. S3.† The devices processed from the binary solvents S-Cl, S-Me and S-DMe showed a weaker dependence of VOC on the light intensity, with a slope around 1.2kT/e, than that of the device fabricated from pure o-xylene, which had a slope of 1.54kT/e.26 It indicates that the strength of recombination was significantly reduced by adding a small amount of additive. Moreover, improved charge transport was shown in Fig. S4† from the improved hole mobility from 3.0 × 10−5 cm2 V−1 s−1 (pure o-xylene) to 2.7 × 10−4 cm2 V−1 s−1 (o-xylene + DMN), determined by the hole-only device using a space charge-limited current (SCLC) model.27 Similarly, the electron mobility increases from 8.2 × 10−5 cm2 V−1 s−1 (pure o-xylene) to 3.8 × 10−4 cm2 V−1 s−1 (o-xylene + DMN), determined from electron-only devices. And the devices processed from non-halogenated solvents (S-DMe) presented similar mobilities to halogenated solvents (S-Cl). The results of the optimal morphology and charge mobility of non-halogenated solvent-cast BHJ films certify the potential of the application of green solvents to replace halogenated ones.
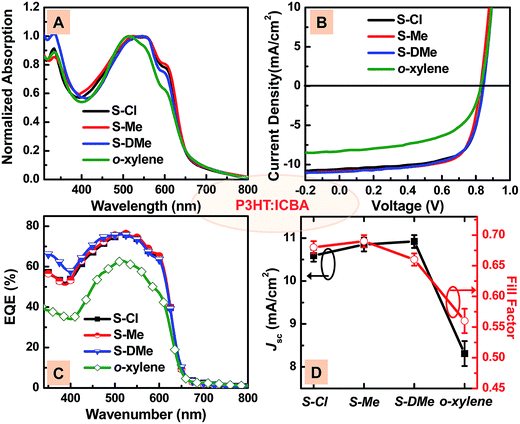
The ladder-type PIDT-FQ-T polymer tends to form a disorder-dominated phase due to the poor packing pattern. Therefore, in order to extend the above findings, the solvent system of S-Me and S-DMe was used in the fabrication of OSCs based on another semi-crystalline conjugated polymer system, P3HT:IC60BA.28 The slower evaporation rate of the high boiling point solvent during the spin coating and drying process may facilitate a re-organization in the blend to a more appropriate phase-separated morphology for charge transport and exciton dissociation. As the P3HT nanocrystal morphology is dictated by the film formation process, the blend morphologies and ultimately the device performance are dependant on the thermodynamics of the solution and kinetics of drying.11 Fig. S5 and S6† illustrated the surface topography and phase images of the P3HT:IC60BA films spun from different solvents. It is clear from these micrographs that the morphology processed from the non-halogenated binary solvent closely resembled that of the film cast from S-Cl, with a similar RMS roughness. For the film cast from o-xylene without additives, the surface was rough with large domains of a hundred nanometers, clearly visible in the micrograph. Before device fabrication, we investigated the absorption features of the casting film dissolved in different solvents and found the absorption shoulder of P3HT (a higher molecular order phase) in S-Cl, S-Me and S-DMe (Fig. S6†), indicating that the degree of molecular order is similar in the as-cast films. Then we further investigated the photovoltaic characteristics of the P3HT:IC60BA BHJ devices at optimal conditions for different solvent systems.
The device of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IC60BA (1
IC60BA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was also explored in the non-halogenated solvents through the same device structure. Fig. 4B presents the J–V curves of the devices and the detailed photovoltaic parameters are summarized in Table 2. The J–V curves show a significant increase in the magnitude of the short circuit current and fill factor with the addition of DMN to the o-xylene solution. Although the S-DMe processed device showed a larger JSC than the S-Cl and S-Me processed one, the device based on the S-Me solvent showed the best performance, with a VOC of 0.84 V, JSC of 10.85 mA cm−2, FF of 0.69, and overall PCE of 6.27%, which is even slightly higher than the performance of the halogenated solvent device (S-Cl) (PCE = 6.12%). The change in the EQE spectra (Fig. 4C) was consistent with the absorption results and confirmed the JSC values. Encouragingly, we demonstrated that our binary non-halogenated solvent can satisfy the requirement of morphology optimization for charge transfer and transport within BHJ films.
1) was also explored in the non-halogenated solvents through the same device structure. Fig. 4B presents the J–V curves of the devices and the detailed photovoltaic parameters are summarized in Table 2. The J–V curves show a significant increase in the magnitude of the short circuit current and fill factor with the addition of DMN to the o-xylene solution. Although the S-DMe processed device showed a larger JSC than the S-Cl and S-Me processed one, the device based on the S-Me solvent showed the best performance, with a VOC of 0.84 V, JSC of 10.85 mA cm−2, FF of 0.69, and overall PCE of 6.27%, which is even slightly higher than the performance of the halogenated solvent device (S-Cl) (PCE = 6.12%). The change in the EQE spectra (Fig. 4C) was consistent with the absorption results and confirmed the JSC values. Encouragingly, we demonstrated that our binary non-halogenated solvent can satisfy the requirement of morphology optimization for charge transfer and transport within BHJ films.
All these results indicate that the non-halogenated solvent system, matched with a principle, could effectively replace the role of the halogenated solvent CB or binary solvent S-Cl. For wide applications, it is important to show that these non-halogenated co-solvents can be generally applicable to other state-of-the-art polymer systems, and we try to apply the solvent mixture of S-Me and S-DMe for the morphological evolution of other highly efficient polymer systems, to realize adaptability on the OSCs. Beside CN, DIO is another very popular solvent additive,13,29 offering selective solubility of active components and extending the drying time, due to its high boiling point. In contrast with CN, DIO could promote the ordering of semi-crystalline conjugated polymers and fullerenes and increase the degree of phase-separation. Nevertheless, from the Hansen solubility parameters, adding a small amount of DIO into DCB will not change the fullerene solubility significantly, since DIO is located far away from PC71BM (Fig. S1†). The solubility of fullerenes in toluene and o-xylene can be obviously improved by incorporating a small amount of MN and DMN additives. In this regard, two representative photovoltaic polymers with varied backbone structures PTB7 (ref. 30) and PBDTTT-C-T31 (Scheme S1†) were selected to demonstrate the halogen-free solvent system. On the basis of previous studies, addition of about 2.5% DIO into the CB (DCB) is needed to realize optimal photovoltaic performance in the two polymer devices. Herein, we compared the device performance of PTB7 and PBDTTT-C-T, by changing the processing solvent. As shown in Fig. S7 and S8,† five types of devices processed respectively by o-xylene, CB + DIO (2.5%), S-Cl, S-Me and S-DMe were fabricated in parallel. When PTB7 and PBDTTT-C-T were used as the donor materials in OSCs, adding CN into the CB host solvent could hardly induce a significant morphology change compared with DIO. From results illustrated in Table 3, it clearly showed that both the non-halogenated solvent system S-Me and S-DMe could retain the performance as CB + DIO halogenated ones with highest PCE over 8%, much better than the S-Cl system. Overall, the performance of the four selected polymer:fullerene systems with different processing solvents is presented in Fig. 5. From the comparison, we found that the non-halogenated binary solvents S-Me and S-DMe have more general applicability for device processing since adding methylnaphthalene (MN or DMN) resulted in an enhanced miscibility of the various active-layer solutions, for better film formation.
| Solventa | VOC (V) | JSC (mA cm−2) | FF | PCEb,c (%) |
|---|---|---|---|---|
| a The concentrations of additives are all 2.5% by volume.b The average device performance was obtained from 20 devices.c The maximum values of the PCEs are provided in parentheses. | ||||
PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1.5) 1.5) |
||||
| CB + DIO | 0.77 ± 0.01 | 15.23 ± 0.23 | 0.62 ± 0.01 | 7.27 ± 0.26 (7.63) |
| S-Cl | 0.79 ± 0.02 | 13.23 ± 0.32 | 0.54 ± 0.02 | 5.64 ± 0.38 (6.28) |
| S-Me | 0.78 ± 0.01 | 14.29 ± 0.25 | 0.61 ± 0.01 | 6.73 ± 0.28 (7.14) |
| S-DMe | 0.77 ± 0.01 | 14.62 ± 0.27 | 0.62 ± 0.01 | 6.96 ± 0.29 (7.32) |
| o-Xylene | 0.79 ± 0.02 | 12.11 ± 0.23 | 0.52 ± 0.02 | 4.97 ± 0.36 (5.56) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1.5) 1.5) |
||||
| CB + DIO | 0.73 ± 0.01 | 16.58 ± 0.24 | 0.66 ± 0.01 | 7.96 ± 0.25 (8.39) |
| S-Cl | 0.74 ± 0.01 | 12.13 ± 0.19 | 0.58 ± 0.02 | 5.24 ± 0.37 (5.86) |
| S-Me | 0.73 ± 0.01 | 16.37 ± 0.30 | 0.62 ± 0.01 | 7.31 ± 0.28 (7.82) |
| S-DMe | 0.74 ± 0.01 | 15.62 ± 0.28 | 0.65 ± 0.01 | 7.51 ± 0.26 (8.01) |
| o-Xylene | 0.72 ± 0.02 | 7.78 ± 0.29 | 0.48 ± 0.02 | 2.71 ± 0.35 (3.21) |
Experimental
Materials
The donor polymers PIDT-FQ-T,21 PBDTTT-C-T31 and PC61BM-COOH32 were synthesized in our laboratory according to previous studies. P3HT, PTB7, PC71BM and IC60BA were purchased from 1-Material Inc. All of the solvents are commercially available products and used without any further purification. All the polymers bearing long-chain alkyl groups have decent solubility even in halogen-free solvents.Device fabrication and characterization
Solar cells were fabricated with an architecture of ITO/ZnO (30 nm)/PC61BM-COOH (SAM)/polymer:fullerene/MoOx (10 nm)/Ag (100 nm). ITO-coated (15 Ω sq−1) glass substrates were cleaned sequentially with a detergent, DI-water, acetone, and isopropanol. Prior to the fabrication of the organic layers, the ITO surface was treated with UV-Ozone. The prepared ZnO sol–gel was spin-coated on the pre-cleaned ITO-coated glass substrate at 4000 rpm. The ZnO films were annealed at 200 °C for 1 h in the air. The PC61BM-COOH (SAM) was deposited on the ZnO surface using a two-step spin process.32 After the surface modification, the substrates were transferred to a nitrogen-filled glove box (<0.1 ppm O2 and H2O) for active-layer coating and electrode formation. The fabrication processes of the control devices of different polymer:fullerene systems are described as follows. PIDT-FQ-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (20 mg mL−1, 1
PC71BM (20 mg mL−1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, w/w) in chlorobenzene/1,8-diiodoctane (97.5
3, w/w) in chlorobenzene/1,8-diiodoctane (97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5% by volume) solution was spin-coated on the ZnO layer at 1000 rpm for 100 s. The films were thermally annealed at 110 °C for 10 min to obtain a film thickness of approximately 90 nm. The blend solution of P3HT and IC60BA derivatives (1
2.5% by volume) solution was spin-coated on the ZnO layer at 1000 rpm for 100 s. The films were thermally annealed at 110 °C for 10 min to obtain a film thickness of approximately 90 nm. The blend solution of P3HT and IC60BA derivatives (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w, 30 mg mL−1) in chlorobenzene/1,8-diiodoctane (97.5
1 w/w, 30 mg mL−1) in chlorobenzene/1,8-diiodoctane (97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5% by volume) was spin-coated on top of the ZnO layer at 800 rpm for 120 s, and then annealed at 150 °C for 10 min. PBDTTT-C-T (10 mg mL−1) and PC71BM (15 mg mL−1) were dissolved in chlorobenzene solution with 2.5 vol% of 1,8-diiodooctane (DIO) as the solvent additive. Similarly, the PTB7
2.5% by volume) was spin-coated on top of the ZnO layer at 800 rpm for 120 s, and then annealed at 150 °C for 10 min. PBDTTT-C-T (10 mg mL−1) and PC71BM (15 mg mL−1) were dissolved in chlorobenzene solution with 2.5 vol% of 1,8-diiodooctane (DIO) as the solvent additive. Similarly, the PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (10
PC71BM (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 mg mL−1) active blend layer was prepared by spin-coating a mixed solvent solution of chlorobenzene/1,8-diiodoctane (97.5
15 mg mL−1) active blend layer was prepared by spin-coating a mixed solvent solution of chlorobenzene/1,8-diiodoctane (97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5% by volume) at 1000 rpm for 120 s. After annealing, 10 nm MoOx and 100 nm Ag were deposited sequentially under high vacuum by thermal evaporation.
2.5% by volume) at 1000 rpm for 120 s. After annealing, 10 nm MoOx and 100 nm Ag were deposited sequentially under high vacuum by thermal evaporation.
All the J–V curves in this study were recorded using a Keithley 2400 source measure unit. The device photocurrent was measured under illumination of a 450 W Thermal Oriel solar simulator (AM 1.5G). The illumination intensity of the light source was accurately calibrated by employing a standard Si photodiode detector equipped with a KG-5 filter, which can be traced back to the standard cell of the National Renewable Energy Laboratory (NREL). The EQE spectra performed here are obtained by IPCE measurement using the combination of a xenon lamp (Oriel, 450 W) as the light source, a monochromator, a chopper with a frequency of 100 Hz, a lock-in amplifier (SR830, Stanford Research Corp), and a Si-based diode (J115711-1-Si detector) for calibration. The absorption and transmission spectra were measured using a Perkin-Elmer Lambda-750 UV-visible spectrophotometer. AFM measurement was carried out using a Digital Instrumental Nanoscope 31, operated in tapping mode. The calculated JSC values obtained by integrating the EQE spectrum under the AM 1.5G illumination condition agreed well with the measured JSC value from the J–V characteristics, and the differences were within 3%.
Conclusions
In summary, we expand the use of the additive strategy for halogenated binary solvents CB + CN (S-Cl) into non-halogenated solvents of toluene + MN (S-Me) and o-xylene + DMN (S-DMe) for the fabrication of highly efficient OSCs. The morphology, charge mobility, and photovoltaic performance of non-halogenated solvent-cast films were systematically investigated and analyzed. We demonstrated that our halogen-free solvent could be applied in the fabrication of OSCs based on a variety of polymers with different crystallinity, and the highest efficiency of over 8% was achieved based on this halogen-free solvent system for PTB7:PC71BM devices. While devices need either CN or DIO to realize optimal photovoltaic performance, devices processed from non-halogenated co-solvents show comparable performances. These results confirmed that the halogen-free solvent system can replace the role of a widely utilized solvent like CB, CB + CN or CB + DIO in the fabrication of environmentally friendly OSCs with high efficiency.Acknowledgements
The authors thank the financial support from the National Natural Science Foundation of China (Grant No. 61504053, 51172103 and 61464007) and Natural Science Foundation of Jiangxi Province, China (20151BAB217023 and 20151BAB207055). Y.-X. X. thanks the financial support from State Key Laboratory of Polymer Materials Engineering (Grant No. sklpme2014-3-16).Notes and references
- A. J. Heeger, Adv. Mater., 2014, 26, 10–27 CrossRef CAS.
- J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C. C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef.
- A. Hübler, B. Trnovec, T. Zillger, M. Ali, N. Wetzold, M. Mingebach, A. Wagenpfahl, C. Deibel and V. Dyakonov, Adv. Energy. Mater., 2011, 1, 1018–1022 CrossRef.
- F. Liu, Y. Gu, X. Shen, S. Ferdous, H.-W. Wang and T. P. Russell, Prog. Polym. Sci., 2013, 38, 1990–2052 CrossRef CAS; Y. Huang, E. J. Kramer, A. J. Heeger and G. C. Bazan, Chem. Rev., 2014, 114, 7006–7043 CrossRef.
- T. R. Andersen, T. T. Larsen-Olsen, B. Andreasen, A. P. L. Böttiger, J. E. Carlé, M. Helgesen, E. Bundgaard, K. Norrman, J. W. Andreasen, M. Jørgensen and F. C. Krebs, ACS Nano, 2011, 5, 4188–4196 CrossRef CAS.
- C. W. Schlenker and M. E. Thompson, Chem. Commun., 2011, 47, 3702–3716 RSC; L. Dou, J. You, Z. Hong, Z. Xu, G. Li, R. A. Street and Y. Yang, Adv. Mater., 2013, 25, 6642–6671 CrossRef CAS.
- R. Giridharagopal and D. S. Ginger, J. Phys. Chem. Lett., 2010, 1, 1160–1169 CrossRef CAS; T.-Y. Chu, S. Alem, S.-W. Tsang, S.-C. Tse, S. Wakim, J. Lu, G. Dennler, D. Waller, R. Gaudiana and Y. Tao, Appl. Phys. Lett., 2011, 98, 253301 CrossRef; K. R. Graham, P. M. Wieruszewski, R. Stalder, M. J. Hartel, J. Mei, F. So and J. R. Reynolds, Adv. Funct. Mater., 2012, 22, 4801–4813 CrossRef.
- G. Nagarjuna and D. Venkataraman, J. Polym. Sci. Part B: Polym. Phys., 2012, 50, 1045–1056 CrossRef CAS.
- K. Tada, Sol. Energy Mater. Sol. Cells, 2013, 108, 82–86 CrossRef CAS.
- K.-S. Chen, H.-L. Yip, C. W. Schlenker, D. S. Ginger and A. K. Y. Jen, Org. Electron., 2012, 13, 2870–2878 CrossRef CAS.
- S. Berson, R. de Bettignies, S. Bailly and S. Guillerez, Adv. Funct. Mater., 2007, 17, 1377–1384 CrossRef CAS.
- C. V. Hoven, X. D. Dang, R. C. Coffin, J. Peet, T. Q. Nguyen and G. C. Bazan, Adv. Mater., 2010, 22, E63–E66 CrossRef CAS.
- J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619–3623 CrossRef CAS PubMed.
- S. J. Lou, J. M. Szarko, T. Xu, L. Yu, T. J. Marks and L. X. Chen, J. Am. Chem. Soc., 2011, 133, 20661–20663 CrossRef CAS PubMed.
- C. M. Hansen, Hansen Solubility Parameters: a User’s Handbook, CRC Press, Boca Raton, FL. USA, 2nd edn, 2007, ch. 1 CrossRef CAS; B. Walker, A. Tamayo, D. T. Duong, X.-D. Dang, C. Kim, J. Granstrom and T.-Q. Nguyen, Adv. Energy Mater., 2011, 1, 221–229 CrossRef CAS.
- C.-C. Chueh, K. Yao, H.-L. Yip, C.-Y. Chang, Y.-X. Xu, K.-S. Chen, C.-Z. Li, P. Liu, F. Huang, Y. Chen, W.-C. Chen and A. K. Y. Jen, Energy Environ. Sci., 2013, 6, 3241–3248 CAS.
- X. Guo, M. Zhang, C. Cui, J. Hou and Y. Li, ACS Appl. Mater. Interfaces, 2014, 6, 8190–8198 CAS.
- W. Zhao, L. Ye, S. Zhang, M. Sun and J. Hou, J. Mater. Chem. A, 2015, 3, 12723–12729 CAS.
- X. Zhang, H. Bronstein, A. J. Kronemeijer, J. Smith, Y. Kim, R. J. Kline, L. J. Richter, T. D. Anthopoulos, H. Sirringhaus, K. Song, M. Heeney, W. Zhang, I. McCulloch and D. M. DeLongchamp, Nat. Commun., 2013, 4, 2238 Search PubMed.
- Y. Kim, S. Cook, S. M. Tuladhar, S. A. Choulis, J. Nelson, J. R. Durrant, D. D. C. Bradley, M. Giles, I. McCulloch, C.-S. Ha and M. Ree, Nat. Mater., 2006, 5, 197–203 CrossRef CAS.
- K. Yao, Y.-X. Xu, F. Li, X. Wang and L. Zhou, Adv. Opt. Mater., 2015, 3, 321–327 CrossRef.
- T. J. Savenije, J. E. Kroeze, X. Yang and J. Loos, Adv. Funct. Mater., 2005, 15, 1260–1266 CrossRef CAS.
- S. K. Hau, H.-L. Yip and A. K. Y. Jen, Polym. Rev., 2010, 50, 474–510 CrossRef CAS.
- S. Nilsson, A. Bernasik, A. Budkowski and E. Moons, Macromolecules, 2007, 40, 8291–8301 CrossRef CAS.
- K. N. Semenov, N. A. Charykov, V. A. Keskinov, A. K. Piartman, A. A. Blokhin and A. A. Kopyrin, J. Chem. Eng. Data, 2010, 55, 13–36 CrossRef CAS.
- S. R. Cowan, A. Roy and A. J. Heeger, Phys. Rev. B: Solid State, 2010, 82, 245207 CrossRef.
- V. Mihailetchi, J. Wildeman and P. Blom, Phys. Rev. Lett., 2005, 94, 126602 CrossRef CAS PubMed.
- G. Zhao, Y. He and Y. Li, Adv. Mater., 2010, 22, 4355–4358 CrossRef CAS PubMed.
- M.-S. Su, C.-Y. Kuo, M.-C. Yuan, U. S. Jeng, C.-J. Su and K.-H. Wei, Adv. Mater., 2011, 23, 3315–3319 CrossRef CAS PubMed.
- Y. Liang, Z. Xu, J. Xia, S. T. Tsai, Y. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, E135–E138 CrossRef CAS PubMed.
- H.-Y. Chen, J. Hou, S. Zhang, Y. Liang, G. Yang, Y. Yang, L. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649–653 CrossRef CAS.
- S. K. Hau, H.-L. Yip, H. Ma and A. K. Y. Jen, Appl. Phys. Lett., 2008, 93, 233304 CrossRef CAS; J. J. Intemann, K. Yao, Y.-X. Li, H.-L. Yip, Y.-X. Xu, P.-W. Liang, C.-C. Chueh, F.-Z. Ding, X. Yang, X. Li, Y. Chen and A. K. Y. Jen, Adv. Funct. Mater., 2014, 24, 1465–1473 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19850j |
| This journal is © The Royal Society of Chemistry 2015 |