Seedless synthesis of gold nanorods using dopamine as a reducing agent†
Abstract
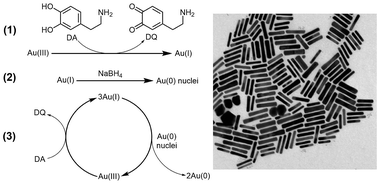
A novel protocol for the seedless synthesis of gold nanorods (AuNRs) using dopamine as a reductant has been developed. We report that the concentration of CTAB can be reduced to 22 mM and that the AuNRs' longitudinal surface plasmon resonance (LSPR) can be tuned from 700 to 1050 nm. The size of the AuNRs can also be tuned from 7 × 30 nm to 20 × 100 nm. The amphiphilicity and the cationic structure of the protonated dopamine enable it to interact favorably with the CTAB bilayer and lead to a high-yield synthesis of AuNRs (80–95%). LSPR peaks, size, and aspect ratio of the synthesized AuNRs can be tuned by adjusting the concentration and ratio of silver ions, CTAB, and dopamine. In addition, this reaction is much faster than previously reported methods requiring only 30 minutes to complete.

 Please wait while we load your content...
Please wait while we load your content...