Direct epitaxial CVD synthesis of tungsten disulfide on epitaxial and CVD graphene†
Abstract
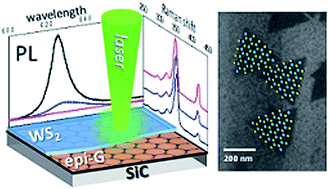
The direct chemical vapor deposition of WS2 by W(CO)6 and elemental sulfur as precursors onto epitaxial-graphene on SiC and CVD-graphene transferred on SiO2/Si substrate is presented. This methodology allows the epitaxial growth of continuous WS2 films with a homogeneous and narrow photoluminescence peak without inducing stress or structural defects in the graphene substrates. The control of the WS2 growth dynamics for providing the localized sulfide deposition by tuning the surface energy of the graphene substrates is also demonstrated. This growth methodology opens the way towards the direct bottom up fabrication of devices based on TMDCs/graphene van der Waals heterostructures.

 Please wait while we load your content...
Please wait while we load your content...