Improvement of heat dissipation in agarose gel electrophoresis by metal oxide nanoparticles
Mohammad Zareia,
Elaheh K. Goharshadiab,
Hossein Ahmadzadeh*a and
Sara Samieea
aDepartment of Chemistry, Ferdowsi University of Mashhad, Mashhad 91779, Iran. E-mail: h.ahmadzadeh@um.ac.ir
bCenter of Nano Research, Ferdowsi University of Mashhad, Mashhad 91779, Iran
First published on 12th October 2015
Abstract
Joule heating is a primary limitation in slab gel electrophoresis which is a gold standard method in biochemistry and biotechnology. In this paper, we introduced an innovative new class of heat transfer nanocomposites engineered by the inclusion of metal oxide nanoparticles (NPs) in a conventional separation medium (gel). The nanocomposite exhibits high thermal conductivity compared to the gel itself. The results suggest a unique correlation between the average particle size and the thermal conductivity of the metal oxide NPs with a resolution improvement in the separation, i.e. a reduction in Joule heating. Ceria, zirconia, tungsten oxide, and lanthania NPs were loaded into agarose gel separately and used as a separation medium for gel electrophoresis. Among the NPs, ceria with the smallest size (5.2 nm) and highest thermal conductivity (17 W m−1 K−1) presented a better performance in reduction of Joule heating. By loading 0.3% (m/v) ceria, zirconia, and tungsten oxide NPs into the agarose gel at 25 °C, the thermal conductivity of the gel increased by 79, 78, and 78%, and resulted in a 22, 18, and 14% reduction in Joule heating (230 V), respectively. The overall separation efficiency and resolution increased for the agarose/zirconia gel as compared with the pure agarose gel. For example, the separation efficiency of the 70 and 80 (bp) peaks increased by 260 and 165%, respectively. Also, the resolution increased from 1.65 for the pure agarose gel to 6.32 for the agarose/zirconia gel.
1. Introduction
Agarose gel electrophoresis (AGE) is an important technique for the separation of biological samples.1 However, it suffers from some physical limitations, leading to band broadening.2 Joule heating is one of the most important factors in the band broadening phenomenon. The heat generated is directly proportional to the separation voltage, electric current, and time. Heat dissipation in slab gel electrophoresis is an important goal to enhance the separation efficiency. Capillary gel electrophoresis (CGE) has been used to resolve this problem by the efficient heat dissipation capability of the capillaries at the expense of a higher cost of the instrument and the loss of the high throughput capability of slab gel electrophoresis. Compared to slab gel electrophoresis, CGE usually needs sophisticated and expensive instruments for the analysis of biomolecules.The addition of nanoparticles (NPs) provides unique opportunities for substantially enhancing the selectivity, efficiency, and stability of analytical techniques.3 The application of NPs has been extensively studied in separation science.4–9 Different types of NPs have been used successfully for separation purposes, such as carbon nanotubes,10 fullerenes,5 silica,6 latex,7 magnetic11 and non-magnetic metal oxides,12 and silver13 and gold NPs.14–17 In addition, polymer-based NPs have been used to coat silica capillaries in capillary electrophoresis.18,19 Surprisingly, very little research has been devoted to the application of NPs in slab gel electrophoresis.8,9
The aims of the present study are to lower Joule heating by using a nanocomposite gel with the incorporation of metal oxide NPs, such as ceria (CeO2), zirconia (ZrO2), tungsten oxide (WO3), and lanthania (La2O3) NPs, in agarose gel and to calculate the separation efficiency. We focused on the separation of two standard DNA samples, 100 bp and 1000 bp mixtures of highly purified DNA fragments. We investigated the influence of NPs on the porosity of agarose gel. The thermal conductivity and the Joule heating were calculated and the temperature profile of the gel was measured in order to understand the influence of the NPs on the thermal properties of the separation matrix.
2. Materials and methods
2.1. Materials
All reagents were of analytical grade. Sodium borate, hydrochloric acid, ammonia, and ethidium bromide were purchased from Merck. Zirconyl nitrate and LaCl3·7H2O were obtained from BDH Prolabo Chemicals. tert-Butyl alcohol and CTAB were purchased from Sigma-Aldrich. Sodium tungstate was purchased from Riedel. Agarose was purchased from KBC. GeneRuler 100 bp and 1 kb DNA standards were obtained from Fermentas and were subjected to electrophoresis without further treatment.2.2. Agarose gel electrophoresis of nucleic acids
All separations were performed on slab gels (1 × 6 × 10 cm). DNA standard samples were analyzed on 1% (m/v) agarose gel. The gels and the buffers contained 2.5 mM sodium borate buffer (pH = 8.2). Nucleic acid samples were applied to the gels in 10 pL 0.01% (m/v) bromophenol blue for DNA. Gel images were analyzed using ImageJ20 and GelAnalyzer21 software.2.3. Preparation of metal oxide NPs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) and washed with deionized water several times. Finally, the products were dried in a vacuum oven at 60 °C overnight.22
000 rpm) and washed with deionized water several times. Finally, the products were dried in a vacuum oven at 60 °C overnight.222.4. Synthesis of nanocomposite hydrogels
In a typical synthesis, 0.20 g of agarose was dissolved in 20.0 mL of sodium borate buffer (2.5 mM) and heated to 80 °C for 10 min. An appropriate amount (0.1–0.3% (m/v)) of each type of NP was rapidly added to the agarose solution. Then, the agarose/NP solution was cooled to room temperature. A temperature probe (Misonix S-400) was used to measure the temperature in different positions of the gel (from cathode to anode).2.5. Characterization methods
X-ray diffraction measurements were performed on a Bruker/D8 Advance diffractometer in the 2θ range from 20° to 80°, in 0.04 degree steps, using graphite monochromatic Cu Kα radiation (λ = 1.541 Å). Transmission electron microscopy (TEM) analysis of the samples was carried out on a LEO 912 AB transmission electron microscope with an electron beam accelerating voltage of 120 kV.3. Results and discussion
3.1. Characterization
Fig. 1 shows the XRD patterns of ceria, zirconia, tungsten oxide, and lanthania NPs. In general, no additional peaks in the XRD patterns of the samples were observed which can be related to the high purity of NPs. Also, the peaks for the NPs are sharp and strong, which indicate the high crystallinity of the samples. The diffraction peaks for the ceria NPs (Fig. 1a) could be indexed to the face-centred cubic phase with the lattice parameters a = b = c = 0.5410 nm.The average crystallite size of the prepared NPs was estimated using the Scherrer formula:25
 | (1) |
Fig. 2 shows TEM images with embedded particle size distributions of ceria, zirconia, tungsten oxide, and lanthania NPs. According to these images, the NPs display uniform morphology with an average size of 5.2, 26, 31.5, and 51.2 nm for the ceria, zirconia, tungsten oxide, and lanthania NPs, respectively.
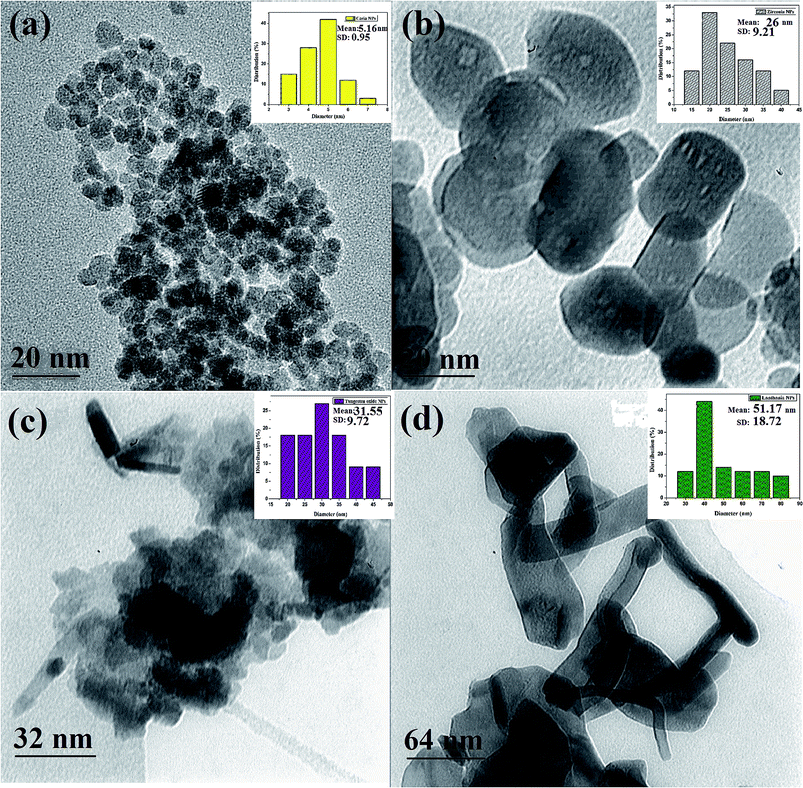 | ||
| Fig. 2 TEM images and particle size distributions of prepared (a) ceria, (b) zirconia, (c) tungsten oxide, and (d) lanthania NPs. | ||
3.2. Joule heating
In order to study the Joule heating effect, an Ohm plot was constructed by applying different voltages across the separation medium and monitoring the generated currents. Fig. 3 shows I–V measurements (Ohm plot) for the agarose gel and the agarose/NP modified gels. The threshold voltage (Ut) above which the current begins to abruptly increase is the maximum separation voltage that should be applied in order to have the shortest separation time. The values of Ut in the pure agarose and the agarose/ceria, agarose/zirconia, agarose/tungsten oxide, and agarose/lanthania gels were estimated to be 170, 200, 190, 180, and 175 V, respectively. At high voltages, the produced heat in the NP embedded gels is less than that of the pure agarose gel (Fig. 3a–d). The heat generated is directly proportional to the separation voltage, U, electric current, I, and time, t:| q = UIt | (2) |
Fig. 3e–h show the calculated Joule heating for the pure agarose gel and the NP embedded gels at different applied voltages. As this figure shows, by addition of the NPs to the agarose gels, the amount of Joule heating decreased. For example, at 150 V, the values of the generated heat were 16.31, 15.39, 16.17, 16.20, 16.45 kJ for the agarose, ceria/agarose, zirconia/agarose, tungsten oxide/agarose, and lanthania/agarose gels, respectively. A significant decrease in Joule heating is seen at higher applied voltages. For example, at 220 V, the values of produced heat were 18.46, 16.42, 16.75, 16.93, and 16.85 kJ in the agarose, ceria/agarose, zirconia/agarose, tungsten oxide/agarose, and lanthania/agarose gels, respectively. In the ceria/agarose gel, the produced heat reduces by 6 and 12% at 150 V and 200 V, respectively.
By increasing the percentage of NP loading, the magnitude of the Joule heating decreases further. By loading 0.3% m/v of NPs, at the applied voltages, the values of Joule heating decreased by 12, 10, 9, and 8% for the agarose/ceria, agarose/zirconia, agarose/tungsten oxide, and agarose/lanthania gels, respectively, compared to that of the pure agarose gels. The order of decrease in Joule heating has good correlation with the average particle size and the thermal conductivity of the NPs. As Table 1 shows, there is a correlation between the average particle size and the thermal conductivity of the metal oxide NPs with the reduction in Joule heating. The ceria NPs, with the smallest size and highest thermal conductivity among the NPs, reduced the Joule heating by 12% when 0.3% m/v was included in the gel. Even at a higher voltage of 230 V, by loading 0.3% m/v ceria NPs into the agarose gel at 25 °C, the thermal conductivity increased by 79%, which resulted in a 22% reduction in Joule heating and an increase in the separation efficiency. Generally, Joule heating increases with applied voltage, but in the NP modified gels, the NPs act as heat sinks and reduce the generated heat. The thermal conductivity of the metal oxide NPs increases significantly with temperature, as well as with the volume fraction of NPs.26 This leads to a reduction of the Joule heating by the agarose/metal oxide NP gel at high voltages.
| NPs | Average particle sizea (nm) | Thermal conductivity of the bulk (W m−1 K−1) | Electrical conductivity of the bulk (S m−1) | Joule heating reduction (%) |
|---|---|---|---|---|
| a Calculated using TEM image.b Not reported. | ||||
| CeO2 | 5.2 | 17 (ref. 31) | 0.044 (ref. 32) | 12 |
| ZrO2 | 26.0 | 4.2 (ref. 33) | 0.86 (ref. 34) | 10 |
| WO3 | 31.5 | 1.6 (ref. 35) | NRb | 9 |
| La2O3 | 51.2 | NR | 10−5 (ref. 36) | 8 |
The temperature difference between the center line and the inside line of the separation medium can be calculated using the Knox equation:27
 | (3) |
 | (4) |
The main criterion is to choose NPs with high thermal conductivities and low electrical conductivities. The reason is simple: we do not wish the NPs to move and/or cause non-uniformities in the electric field, i.e. NPs with low electrical conductivities. At the same time, we wish the NPs to act as heat sinks to effectively dissipate heat and lower Joule heating, i.e. NPs with high thermal conductivities. Based on this criterion, ceria, zirconia, tungsten oxide, and lanthania NPs were chosen (Table 1).
There is an important disadvantage of using CNTs in the separation medium. CNTs have high thermal conductivity (3500 W m−1 K−1)37 and high electrical conductivity (106 to 107 S m−1).38 CNTs move when a separation voltage is applied. That causes the depletion of CNTs on one side of the gel and accumulation on the other side. If a voltage is applied for a long time, the CNTs keep moving and might egress from the other side of the gel. The Joule heating would be lower on the side where the CNTs have migrated to, and higher on the depleted side. In this work, we chose metal oxide NPs since they have high thermal conductivity but low electrical conductivity. Hence, metal oxide NPs increase the thermal conductivity of the separation medium, without generation of a concentration gradient across the gel.
The temperature difference between the center and the edges of the gels measured using a temperature probe and calculated using the Knox equation (eqn (3)) is shown in Fig. 4. The temperature gradient in the agarose/metal oxide NPs is smaller than that of pure agarose due to the higher thermal conductivity of the NPs (Fig. 4). At 200 V, the temperature gradient across the agarose/ceria, agarose/zirconia, agarose/tungsten oxide, and agarose/lanthania gels were 0.20, 0.35, 0.60, and 1.00 °C, respectively, when the loading amount of NPs was 0.1% m/v, while its value was 2.5 °C in pure agarose gel. For a loading amount of 0.3% m/v, the temperature gradient across the agarose/ceria, agarose/zirconia, agarose/tungsten oxide, and agarose/lanthania gels was 0.17, 0.28, 0.41, and 0.77 °C, respectively. By increasing the percentage of NP loading from 0.1 to 0.3% m/v, the temperature gradient in the gel reduced further. This is related to the higher values of thermal conductivity of the gel/NPs than that of the pure agarose gel.
There are no theoretical formulae to predict the thermal conductivity of suspensions of NPs, so-called nanofluids, satisfactorily.39 The Maxwell model,40 a traditional model for thermal conductivity, has been proposed for solid–liquid mixtures:
 | (5) |
The thermal conductivity of agarose/ceria, agarose/zirconia, and agarose/tungsten oxide was calculated according to the Maxwell model as 1.0010, 1.0003, and 1.0061, respectively, for 0.3% (m/v) of the NPs. This is equivalent to an enhancement in thermal conductivity of 79% for agarose/ceria, 78% for agarose/zirconia, and 78% for agarose/tungsten oxide gels compared with that of the agarose gel. Hence, the NPs act as heat sinks to lower the produced heat. After careful analysis with respect to the Joule heating and thermal conductivity measurements, the agarose/ceria and agarose/zirconia nanocomposite gels were chosen as separation media for the separation of DNA samples.
3.3. Separation of standard DNA samples by the NP modified gels
The separation panels and electropherograms of standard DNA samples (a mixture of 10 highly purified DNA fragments (100 bp: 10–100 bp and 1 kb: 100–1000 bp)) on agarose gel and the agarose gel/NPs are shown in Fig. 5. The separations were performed at different voltages (170, 210, and 200 V for agarose, agarose/ceria, and agarose/zirconia, respectively) chosen based on the threshold voltage for the initiation of Joule heating for agarose and agarose/NPs. Also, we compared the performance of the agarose/NPs with that of the pure gel for separation at high voltages. For the separation of the 1 kb DNA sample (Fig. 5a–c), the number of separated DNA fragments in the range of 700–1000 bp increased for agarose/ceria and agarose/zirconia compared to those of the pure agarose gel. Also, the separation time decreased in the agarose gel/NPs compared to that in the pure agarose gel because of the higher applied voltages. In the separation of the 100 bp DNA sample, the number of separated DNA fragments increased in agarose/ceria compared to that in the pure agarose gel (Fig. 5d–f).The efficiency of the electrophoresis can be evaluated by the number of theoretical plates, N:2
 | (6) |
 | (7) |
| Peak (bp) | N | ||
|---|---|---|---|
| Agarose gel | Ceria NPs (0.3% m/v) | Zirconia NPs (0.3% m/v) | |
| 100 | 354.56 | 101.75 | 176.32 |
| 200 | 610.78 | 132.65 | 345.36 |
| 300 | 400.26 | 124.32 | 246.32 |
| 400 | 173.73 | 1418.24 | 110.65 |
| 500 | 138.50 | 110.78 | 121.32 |
| 600 | 311.60 | 632.12 | 187.32 |
| 700 | 16.78 | 1418.81 | 26.32 |
| 800 | — | 1246.50 | 45.29 |
| 900 | 2.82 | 554.00 | 72.32 |
| 1000 | — | 640.12 | 69.31 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 10 | 694.93 | 265.12 | 2650.97 |
| 20 | 608.00 | 285.63 | 4989.56 |
| 30 | 384.56 | 177.32 | 3462.50 |
| 40 | 126.70 | 101.32 | 2681.36 |
| 50 | 122.65 | 234.06 | 3512.36 |
| 60 | 77.90 | 138.62 | 2216.21 |
| 70 | 54.10 | 354.56 | 482.59 |
| 80 | 43.43 | 635.32 | 384.70 |
| 90 | 73.12 | 452.31 | 670.34 |
| 100 | 46.32 | 532.15 | 571.25 |
The resolution between two peaks in the electropherogram can be calculated as:2
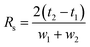 | (8) |
Close examination of the bands shows broad peaks for the agarose gel, whereas these broad peaks split into partially resolved peaks in the agarose/zirconia gel. This shows an enhancement in resolution when the metal oxide NPs are embedded in the agarose gel. For separation of 1 kb and 100 bp DNA fragments using agarose/zirconia gel electrophoresis, the resolution for all adjacent pairs was calculated. The results are tabulated in Table 3 and shown in Fig. 5. The separation parameters in agarose gel/zirconia and the number of separated DNA fragments significantly increased as compared to those of the pure gel. The number of separated DNA fragments for separation at 210 V increased in agarose gel/ceria (0.3% m/v) compared to that of the pure gel. There is a significant decrease in the analysis time at higher voltages with the NP modified gel. For example, the separation time of DNA 100 (bp) by agarose, agarose/ceria, and agarose/zirconia is 30, 17, and 22 min, and for DNA 1 (kb) it is 30, 17, and 20 min, respectively. Also, the sharpness of the peaks increased in agarose gel/ceria. The number of peaks for the separation of long-stranded DNA fragments (100 bp: 70–100 bp, 1000 bp: 700–1000 bp) increased in both agarose/ceria and agarose/zirconia gels compared to those of the agarose gel.
| Peak (bp) | Rs | ||
|---|---|---|---|
| Agarose gel | Ceria NPs (0.3% m/v) | Zirconia NPs (0.3% m/v) | |
| 100–200 | 2.54 | 1.57 | 1.09 |
| 200–300 | 2.00 | 1.11 | 1.62 |
| 300–400 | 2.12 | 1.20 | 1.33 |
| 400–500 | 1.33 | 1.60 | 1.25 |
| 500–600 | 1.66 | 0.66 | 0.57 |
| 600–700 | 0.5 | 1.32 | 0.65 |
| 700–800 | — | 4.12 | 0.55 |
| 800–900 | — | 2.32 | 1.06 |
| 900–1000 | — | 4.65 | 1.12 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 10–20 | 2.58 | 1.55 | 3.33 |
| 20–30 | 2.10 | 1.50 | 3.56 |
| 30–40 | 2.15 | 1.62 | 3.00 |
| 40–50 | 2.32 | 1.55 | 4.12 |
| 50–60 | 1.71 | 1.12 | 4.28 |
| 60–70 | 1.62 | 1.09 | 5.12 |
| 70–80 | 1.65 | 2.59 | 6.32 |
| 80–90 | 1.81 | 2.01 | 2.31 |
| 90–100 | 0.65 | 2.25 | 1.23 |
The effective separation of long-stranded DNA fragments is important in comprehensive genomic analysis of DNA samples and is usually performed by pulsed field gel electrophoresis.45 Unfortunately, pulsed field gel electrophoresis is slow and separation times are in the range of several hours to several days. Although capillary electrophoresis (CE) with entangled polymer46 solutions has been used for the separation of DNA molecules of different sizes,47–49 CE is not efficient for separation of long-stranded DNA due to the requirement of sophisticated and expensive instruments. Ultradilute polymer solutions,50,51 pulsed-field CE,52–54 entropy trap arrays,55,56 asymmetric obstacle arrays,57 and a DNA prism58 have been developed to overcome the problem of time delays in CE.
Huang et al.59 separated long double-stranded DNA (dsDNA) molecules using nanoparticle-filled capillary electrophoresis. They used gold nanoparticles (GNPs) modified with poly(ethylene oxide) (PEO) to form gold nanoparticle/polymer composites (GNPPs). GNPPs are heavy and electrically neutral: thus, they slow down DNA molecule migration in electrophoresis. Polymer solutions such as PEO and GNPPs provide greater efficiency and shorter migration times for separation of long dsDNA. Polymer chains could attach to the surfaces of NPs through their hydrophobic parts, which leaves their hydrophilic groups available to interact with the polar dispersion media.
NPs can be wrapped by long single- and double-stranded DNA (ssDNA and dsDNA). ssDNA and dsDNA have different affinities toward NPs.60 The essential difference in adsorption behavior between ssDNA and dsDNA may be attributed to their different electrostatic properties. Also, ssDNA has a flexible structure and uncoils in solution under the influence of an applied voltage. In contrast, dsDNA has a stable and rigid structure.61 For example, long ssDNA can wrap around CNTs.62,63 Hence, it is plausible to say that a large ssDNA fragment may adsorb onto NPs and may be trapped by NPs.
Polymer chains are adsorbed on the surface of metal oxide NPs to form an NP/polymer composite. The composite is heavy and electrically neutral, hence it slows the migration of DNA molecules. Long DNA fragments have a greater probability to interact with more than one NP, depending on the DNA size and conformation. The drag force is stronger for long DNA fragments as compared to short ones. As a result, the mobility of long DNA is smaller than that of a short one. Fig. 5 shows that for the agarose/ceria gel, the resolution and efficiency of DNAs with higher molecular weights (700–1000 bp) are greater as compared with lower molecular weight DNA (100–600 bp), which could be explained by the interaction of long stranded DNAs with the NPs' surfaces.
Incorporation of NPs into the separation medium could change the physicochemical behavior and thermal conductivity of the separation medium.8
3.4. Influence of the NP concentration
In order to understand the relationship between the NP concentration and the degree of improvement in the separation parameters, three different concentrations of NPs (0.1, 0.3, and 0.5% m/v) were incorporated into agarose gel. The optimum separation efficiency was obtained for 0.3% m/v NPs. However, when the concentration of the NPs is higher than 0.3% m/v, no significant improvement in separation parameters was achieved. An excess NP concentration will cause interference with the UV light produced by the gel documentation system, which increases the background of images, and lowers the S/N ratio of the separation profiles.3.5. Influence of NPs on gel porosity
In order to understand the influence of NP incorporation on the porosity of the agarose gel, the porosity was calculated by measuring the density of dry and wetted gels. The overall gel porosity was calculated using the following formula:64
 | (9) |
The wetted density is the ratio of the weight to volume of a gel which was saturated with a wetting liquid for 24 h at room temperature. Table 4 shows that the porosity in water increases with the amount of NP filler. For example, in a gel containing 0.1 and 0.3% m/v ceria NPs, the porosity increased by 2 and 3% with increasing filler content. The porosity of the gel was increased by increasing the amount of NPs. In general, the sharpness and resolution of all defined bands increased in the NP modified gels because the NPs alter both the chemical composition and thermal conductivity of the polymer matrix.
| Matrix | Porosity enhancement | |
|---|---|---|
| 0.1% m/v | 0.3% m/v | |
| Agarose/ceria | 2 | 3 |
| Agarose/tungsten oxide | 1 | 2 |
| Agarose/zirconia | 2 | 4 |
By introducing NPs into the matrix, the pore size distribution of the matrix changes with respect to the pure gel. The inclusion of NPs has been shown to cause nucleation, which reduces the pore size of the polymer matrix.65 Also, as the size of the NPs decreases, the pore density increases compared to that of the gel.
4. Conclusions
Metal oxide NPs were embedded into an agarose gel to increase the separation efficiency by reducing Joule heating and lowering band broadening. The influence of the NPs on Joule heating was successfully evaluated. A reduction in Joule heating was observed by dispersing the NPs in agarose gel. The results showed a unique correlation between the average particle size and the thermal conductivity of the metal oxide NPs with a reduction in the amount of generated heat. Among the metal oxide NPs studied, ceria with the smallest size (5.2 nm) and highest thermal conductivity (17 W m−1 K−1) presented a better performance in lowering the Joule heating.The optimum separation efficiency was obtained for 0.3% m/v NPs. For example, by loading 0.3% m/v ceria NPs in agarose gel at 25 °C, the thermal conductivity increased by 79%, which resulted in a 22% reduction in Joule heating and an increase in the separation efficiency. Finally, this paper presents the potential to improve the separation efficiency and resolution of other biomolecules using slab gel electrophoresis.
Acknowledgements
The authors acknowledge the ATF committee and Ferdowsi University of Mashhad for supporting this project (3/23032). The authors also thank Dr Razieh Jalal and Dr Ahmad Reza Bahrami for their generous donation of DNA standards.Notes and references
- B. D. Hames, Gel Electrophoresis of Proteins: A Practical Approach, Oxford University Press, 1998 Search PubMed.
- R. Kuhn and S. Hoffstetter-Kuhn, Capillary Electrophoresis: Principles and Practice, Springer-verlag, Berlin, 1993 Search PubMed.
- C. R. Martin and D. T. Mitchell, Anal. Chem., 1998, 70, 322A–327A CrossRef CAS PubMed.
- L. Yang, E. Guihen, J. D. Holmes, M. Loughran, G. P. O'Sulliva and J. D. Glennon, Anal. Chem., 2005, 77, 1840–1846 CrossRef CAS PubMed.
- M. R. Ivanov and J. H. Amanda, Analyst, 2011, 136, 54–63 RSC.
- L. Tang, X. Wang, B. Guo, M. Ma, B. Chen, S. Zhan and S. Yao, RSC Adv., 2013, 3, 15875–15886 RSC.
- D. Xiao, C. Zhang, D. Yuan, J. He, J. Wu and H. He, RSC Adv., 2014, 4, 64843–64854 RSC.
- M. Zarei, H. Ahmadzadeh and E. K. Goharshadi, Analyst, 2015, 140, 4434–4444 RSC.
- M. Zarei, H. Ahmadzadeh, E. K. Goharshadi and A. Farzaneh, Anal. Chim. Acta, 2015, 887, 245–252 CrossRef CAS PubMed.
- H. Luo, Z. Shi, N. Li, Z. Gu and Q. Zhuang, Anal. Chem., 2001, 73, 915–920 CrossRef CAS.
- A. Manbohi, S. H. Ahmadi and V. Jabbari, RSC Adv., 2015, 5, 57930–57936 RSC.
- A. Kolmakov and M. Moskovits, Annu. Rev. Mater. Res., 2004, 34, 151–180 CrossRef CAS.
- A. D. McFarland and R. P. van Duyne, Nano Lett., 2003, 3, 1057–1062 CrossRef CAS.
- E. Guihen and J. D. Glennon, Anal. Lett., 2003, 36, 3309–3336 CrossRef CAS PubMed.
- T. B. L. Kist and M. Mandaji, Electrophoresis, 2004, 25, 3492–3497 CrossRef CAS PubMed.
- V. Sharma, K. Park and M. Srinivasarao, Mater. Sci. Eng., 2009, 65, 1–38 Search PubMed.
- C.-S. Wu, F.-K. Liu and F.-H. Ko, Anal. Bioanal. Chem., 2011, 399, 103–118 CrossRef CAS PubMed.
- G. Kleindienst, C. G. Huber, D. T. Gjerde, L. Yengoyan and G. K. Bonn, Electrophoresis, 1998, 19, 262–269 CrossRef CAS PubMed.
- C. G. Huber, A. Premstaller and G. Kleindienst, J. Chromatogr. A, 1999, 849, 175–189 CrossRef CAS.
- M. D. Abràmoff, P. J. Magalhães and S. J. Ram, Biophotonics Int., 2004, 11, 36–43 Search PubMed.
- I. Lazar and I. Lazar, Gel Analyzer 2010a: Freeware 1D Gel Electrophoresis Image Analysis Software, 2012 Search PubMed.
- E. K. Goharshadi, S. Samiee and P. Nancarrow, J. Colloid Interface Sci., 2011, 356, 473–480 CrossRef CAS PubMed.
- E. K. Goharshadi and M. Hadadian, Ceram. Int., 2012, 38, 1771–1777 CrossRef CAS PubMed.
- E. K. Goharshadi, T. Mahvelati and M. Yazdanbakhsh, J. Iran. Chem. Soc., 2015, 1–8 Search PubMed.
- P. Scherrer, Nachr. Ges. Wiss. Goettingen, Geschaeftliche Mitt., 1918, 2, 96–100 Search PubMed.
- C. H. Li and G. Peterson, J. Appl. Phys., 2006, 99, 084314 CrossRef PubMed.
- J. Knox and I. Grant, Chromatographia, 1987, 24, 135–143 CAS.
- M. Zhang, Z. Che, J. Chen, H. Zhao, L. Yang, Z. Zhong and J. Lu, J. Chem. Eng. Data, 2010, 56, 859–864 CrossRef.
- G. Huang, Y. Zhang, J. Ouyang, W. R. Baeyens and J. R. Delanghe, Anal. Chim. Acta, 2006, 557, 137–145 CrossRef CAS PubMed.
- Y. Guo, L. Huang, W. R. Baeyens, J. R. Delanghe, D. He and J. Ouyang, Nano Lett., 2009, 9, 1320–1324 CrossRef CAS PubMed.
- M. Khafizov, I. W. Park, A. Chernatynskiy, L. He, J. Lin, J. J. Moore, D. Swank, T. Lillo, S. R. Phillpot and A. El-Azab, J. Am. Ceram. Soc., 2014, 97, 562–569 CrossRef CAS PubMed.
- J. L. Rupp and L. J. Gauckler, Solid State Ionics, 2006, 177, 2513–2518 CrossRef CAS PubMed.
- J. F. Bisson, D. Fournier, M. Poulain, O. Lavigne and R. Mévrel, J. Am. Ceram. Soc., 2000, 83, 1993–1998 CrossRef CAS PubMed.
- G. Cao, J. Appl. Electrochem., 1994, 24, 1222–1227 CrossRef CAS.
- J. Liqiang, Q. Yichun, W. Baiqi, L. Shudan, J. Baojiang, Y. Libin, F. Wei, F. Honggang and S. Jiazhong, Sol. Energy Mater. Sol. Cells, 2006, 90, 1773–1787 CrossRef PubMed.
- V. Lazarev, V. Krasov and I. Shaplygin, Electrical Conductivity of Oxide Systems and Film Structures, Nauka, Moscow, 1979 Search PubMed.
- E. Pop, D. Mann, Q. Wang, K. Goodson and H. Dai, Nano Lett., 2006, 6, 96–100 CrossRef CAS PubMed.
- Q. Li, Y. Li, X. Zhang, S. B. Chikkannanavar, Y. Zhao, A. M. Dangelewicz, L. Zheng, S. K. Doorn, Q. Jia and D. E. Peterson, Adv. Mater., 2007, 19, 3358–3363 CrossRef CAS PubMed.
- E. Goharshadi, H. Ahmadzadeh, S. Samiee and M. Hadadian, Phys. Chem. Res., 2009, 1, 1–33 Search PubMed.
- R. Kochetov, A. Korobko, T. Andritsch, P. Morshuis, S. Picken and J. Smit, J. Phys. D: Appl. Phys., 2011, 44, 395401 CrossRef.
- M. Abareshi, E. K. Goharshadi, S. M. Zebarjad, H. K. Fadafan and A. Youssefi, J. Magn. Magn. Mater., 2010, 322, 3895–3901 CrossRef CAS PubMed.
- J. Giddings, Unified Separation Science, John Wiley and Sons, New York, 1991, ch. 8, p. 166 Search PubMed.
- Y. Ito and J. Cazes, Encyclopedia of Chromatography, Taylor & Francis, 2001 Search PubMed.
- J. C. Reijenga, in Encyclopedia of Chromatography, Taylor & Francis, 2nd edn, 2005, p. 121 Search PubMed.
- D. C. Schwartz and C. R. Cantor, Cell, 1984, 37, 67–75 CrossRef CAS.
- K. D. Cole and C. M. Tellez, Biotechnol. Prog., 2002, 18, 82–87 CrossRef CAS PubMed.
- Y. Kim and E. S. Yeung, J. Chromatogr. A, 1997, 781, 315–325 CrossRef CAS.
- F. Han, B. H. Huynh, Y. Ma and B. Lin, Anal. Chem., 1999, 71, 2385–2389 CrossRef CAS PubMed.
- H. Zhou, A. W. Miller, Z. Sosic, B. Buchholz, A. E. Barron, L. Kotler and B. L. Karger, Anal. Chem., 2000, 72, 1045–1052 CrossRef CAS.
- A. E. Barron, D. S. Soane and H. W. Blanch, J. Chromatogr. A, 1993, 652, 3–16 CrossRef CAS.
- B. Braun, H. W. Blanch and J. M. Prausnitz, Electrophoresis, 1997, 18, 1994–1997 CrossRef CAS PubMed.
- W. Volkmuth and R. Austin, Nature, 1992, 358, 600–602 CrossRef CAS PubMed.
- Y. Kim and M. D. Morris, Anal. Chem., 1995, 67, 784–786 CrossRef CAS.
- H. Oana, M. Ueda and K. Yoshikawa, Electrophoresis, 1997, 18, 1912–1915 CrossRef CAS PubMed.
- J. Han and H. Craighead, Science, 2000, 288, 1026–1029 CrossRef CAS.
- J. Han and H. G. Craighead, Anal. Chem., 2002, 74, 394–401 CrossRef CAS.
- C.-F. Chou, O. Bakajin, S. W. Turner, T. A. Duke, S. S. Chan, E. C. Cox, H. G. Craighead and R. H. Austin, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 13762–13765 CrossRef CAS.
- L. R. Huang, J. O. Tegenfeldt, J. J. Kraeft, J. C. Sturm, R. H. Austin and E. C. Cox, Nat. Biotechnol., 2002, 20, 1048–1051 CrossRef CAS PubMed.
- M.-F. Huang, Y.-C. Kuo, C.-C. Huang and H.-T. Chang, Anal. Chem., 2004, 76, 192–196 CrossRef CAS PubMed.
- H. Deng, X. Zhang, A. Kumar, G. Zou, X. Zhang and X.-J. Liang, Chem. Commun., 2012, 49, 51–53 RSC.
- H. Li and L. Rothberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14036–14039 CrossRef CAS PubMed.
- W. Zhao, Y. Gao, M. A. Brook and Y. Li, Chem. Commun., 2006, 3582–3584 RSC.
- F. Zhao, H. Cheng, Y. Hu, L. Song, Z. Zhang, L. Jiang and L. Qu, Sci. rep., 2014, 4, 1–7 Search PubMed.
- K. de Sitter, C. Dotremont, I. Genné and L. Stoops, J. Membr. Sci., 2014, 471, 168–178 CrossRef CAS PubMed.
- K. Goren, L. Chen, L. S. Schadler and R. Ozisik, J. Supercrit. Fluids, 2010, 51, 420–427 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |












