Efficient synthesis of xanthene derivatives using carboxyl functionalized graphene quantum dots as an acidic nano-catalyst under microwave irradiation†
Abstract
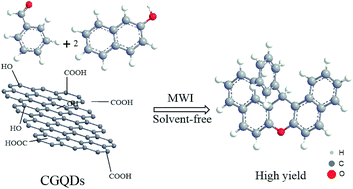
Carboxyl functionalized graphene quantum dots (CGQDs) were applied as a highly efficient and green acidic catalyst for the coupling reaction of 2-naphthole and benzaldehyde derivatives for the preparation of 14H-dibenzo xanthene derivatives, under solvent-free microwave irradiation conditions. The structures of the 14H-dibenzo xanthene derivatives were confirmed by FT-IR, 1H-NMR and 13C-NMR spectroscopic techniques. The structure of catalyst was also confirmed by FT-IR, XRD, TEM and PL techniques. In order to demonstrate the positive impact of the CGQDs catalyst, the reaction times and yields of the products were compared with similar products which have been reported previously. 14H-Dibenzo xanthene derivatives were synthesized in a simple procedure with good to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...