Partial intercalative binding of the food colorant erythrosine to herring sperm DNA
Langhong Wang†
a,
Mo Tao†a,
Guowen Zhang*a,
Song Lia and
Deming Gongb
aState Key Laboratory of Food Science and Technology, Nanchang University, Nanchang 330047, China. E-mail: gwzhang@ncu.edu.cn; Fax: +8679188304347; Tel: +8679188305234
bSchool of Biological Sciences, The University of Auckland, Auckland 1142, New Zealand
First published on 11th November 2015
Abstract
Erythrosine (Ery) is an artificial colorant extensively used in the food industry, but may have a potential safety risk. In this study, the characteristics of the interaction in vitro between Ery and herring sperm DNA (hsDNA) were determined by multi-spectroscopic techniques and docking simulations. The multivariate curve resolution-alternating least squares chemometrics approach was used to process the expanded UV-vis spectral data matrix, and both the equilibrium concentration profiles and the pure spectra for the components (Ery, hsDNA and Ery–hsDNA complex) extracted from the highly overlapping composite response were obtained simultaneously to monitor the Ery–hsDNA interaction process. Partial intercalation as the dominant mode of Ery binding to hsDNA was found based on the hypochromism and red shift effect of Ery, stronger double DNA effect and decrease in hsDNA viscosity. The Fourier transform infrared and nuclear magnetic resonance spectra showed that the benzene ring of Ery most likely inserted into the guanine and cytosine bases of hsDNA, and molecular docking predicted and visualized the specific binding. The circular dichroism and DNA cleavage assays suggested that Ery at high concentrations may perturb B-DNA conformation and cause slight DNA cleavage. This study has provided insights into the binding mechanism of Ery with hsDNA and potential hazards of the colorant.
1. Introduction
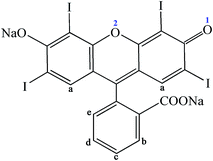
Erythrosine (Ery, Fig. 1), a synthetic food dye with high water solubility, is extensively used in food, such as candies, chocolates, garlic sausages, cake decorating, cocktails and ice creams.1 This synthetic colorant can effectively improve the appearance and color of food products which may enhance consumer attractiveness. Compared to many natural dyes, Ery has more applications because of its high stability in light, color uniformity, low microbiological contamination and relatively lower production costs.2,3 However, Ery may have a potential safety risk when it is excessively consumed.4,5 It was reported that Ery induced DNA damage in the gastrointestinal organs like glandular stomach and colon at a low dose,6 and affected thyroid activity due to its high iodine content.7 Moreover, Ery showed a high cytotoxicity and cytostaticity with its concentrations at 1, 2, 4 and 8 mM.8 Excessive consumption of this dye was considered to have carcinogenic potency.9 In the light of these health hazards, the use of this artificial colorant must be strictly limited by laws, regulations and acceptable daily intake values.DNA is frequently the main molecular target for harmful chemicals, such as organic dyes, heavy metals, pesticides, etc., which enter into the body by inhalation, ingestion or skin.10 They can interact with DNA either covalently or non-covalently, which may interfere with transcription and/or DNA replication mechanisms, alter DNA structure and function, and even trigger processes like cell apoptosis and canceration.11 Generally, the small molecules bind to DNA primarily through three modes: intercalation between stacked base pairs, non-covalent groove binding and electrostatic binding with the negatively charged nucleic acid sugar–phosphate structure.12 In the past few years, more and more researchers investigated the interaction of small molecules with DNA such as drugs, metal complexes and organic dyes as it may provide information on the design and screening of novel and more efficient drugs targeting DNA.13–15 In recent years, the interactions of synthetic food colorants with DNA have attracted much attention due to the potential hazards of some food colorants. Basu and Kumar16 reported that carmoisine bound to herring testes DNA via a groove binding mode and this colorant induced moderate conformational changes in the B-form structure of DNA in vitro. Shahabadi et al.17 found that quinoline yellow strongly competed with Hoechst 33258 for the groove binding site of calf thymus DNA (ctDNA), but it did not cause any significant ctDNA cleavage. Kashanian et al.18,19 have investigated the interactions of the food dyes sunset yellow and tartrazine with ctDNA, and found that both of them bound to ctDNA via groove binding mode. Based on these results, the authors believed that more attention should be paid to prevent children from consuming large amounts of food containing the two colorants. Ery was reported to interact with ctDNA to form a new product, resulting in hypochromism of Ery in the band at 527 nm and hyperchromism of ctDNA in the band at 260 nm along with blue shift of 2–3 nm.8 However, more in-depth studies concerning the binding mode, binding affinity, interaction mechanism and structural change of DNA binding have not been reported, which attracts our interest.
Humans are exposed to different synthetic food additives through the food chain. These chemicals may exhibit their toxicity by interacting with the DNA and affecting DNA structure and function after entering human body. Exploring the binding mechanism of food additives with DNA in vitro is helpful for the assessment of their potential toxicological effects. Therefore, this work was aimed to further investigate the characteristics of binding between Ery and herring sperm DNA (hsDNA) in vitro and the conformational changes of hsDNA binding under simulated physiological conditions (Tris–HCl buffer, pH 7.4) by using multi-spectroscopic methods including fluorescence, UV-vis absorption, circular dichroism (CD), Fourier transform infrared (FT-IR), nuclear magnetic resonance (1H NMR) spectroscopy, hsDNA melting and viscosity analyses coupled with molecular modeling studies. Moreover, the multivariate curve resolution alternating least squares (MCR-ALS) algorithm, a chemometrics method, was used to analyze the complicated UV-vis absorption spectral data matrix collected from the Ery–hsDNA interaction to understand the kinetic process of the binding reaction. Additionally, the interaction between Ery and supercoiled pUC18 plasmid DNA was studied by gel electrophoresis experiments to further evaluate the cleavage effect of Ery on the DNA structure. This study is expected to provide further understanding on the binding mechanism of Ery with hsDNA and offer useful information on the potential hazard of the food colorant.
2. Materials and methods
2.1. Chemicals
Ery (analytical grade) was obtained from Aladdin Chemical Co. (Shanghai, China), and its stock solution (5.0 × 10−3 mol L−1) was prepared in ultrapure water and kept in dark to prevent any light-induced photochemical changes. HsDNA was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), and its stock solution was obtained by dissolving an appropriate amount of hsDNA in a 0.1 mol L−1 NaCl solution and stored at 4 °C. The purity of the final DNA stock solution was checked by determining the absorption ratio at 260 and 280 nm (A260/A280), and the ratio gave a value of 1.83, indicating that the hsDNA was free from protein contamination.20 The concentration of hsDNA in stock solution was determined to be 1.28 × 10−3 mol L−1 by UV-vis absorption at 260 nm using a molar absorption coefficient ε260 = 6600 L mol−1 cm−1.21 Supercoiled pUC18 plasmid DNA was purchased from Beijing Solarbio Science and Technology Ltd (Beijing, China). All stock solutions were diluted to the required concentrations with 0.05 mol L−1 Tris–HCl buffer, pH 7.4. All other chemicals were of analytical reagent grade, and ultrapure water was produced by Millipore Simplicity water purification system (Millipore, Molsheim, France) and used throughout the whole experiment.2.2. Methods
| F0/F = 1 + KSV[Q] | (1) |
The association constant (Ka) of Ery–hsDNA interaction was calculated by the modified Stern–Volmer equation:23
 | (2) |
The preparation of single-strand DNA (ssDNA) was prepared by the method reported previously.24 The quenching effects of ss hsDNA and double-stranded hsDNA (ds hsDNA) were compared by gradually adding ss hsDNA or ds hsDNA solution to Ery solution at a constant concentration of 5.0 × 10−5 mol L−1. The corresponding fluorescence intensity was recorded, and then the quenching constants were determined.
The RLS spectra were obtained by progressively titrating hsDNA to the fixed amount of Ery, and then synchronous scanning on the spectrofluorometer (Δλ = 0 nm) with the wavelength range of 250–700 nm at room temperature.
The multivariate calibration algorithm MCR-ALS has been described in detail previously.30–32 Therefore, a short specification was given here. The bilinear decomposition of the augmented matrix D is performed according to the following expression:
| D = C × ST + E | (3) |
The related bilinear model for MCR-ALS analysis could be expressed by the following equation:
 | (4) |
3. Results and discussion
3.1. Spectral decomposition: application of MCR-ALS
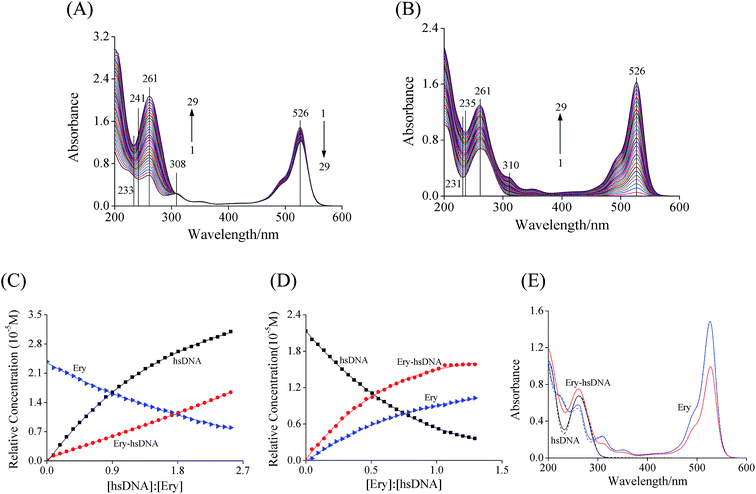
As shown in Fig. 2A, the UV-vis absorption spectra of Ery exhibited three absorption bands at 526, 308 and 261 nm respectively. When hsDNA was added to the solution, the absorption intensity of Ery at 526 nm decreased gradually accompanied by a remarkable increase at 261 nm (corresponding to curves from 1 to 29), and an isobestic point at 308 nm occurred. On the other hand, the maximum absorption peak of hsDNA at 261 nm (curve 1) increased gradually and two new absorption bands at 310 and 526 nm (Ery characteristic absorbance peak) were observed with the continuous addition of Ery (curves 1 to 29, Fig. 2B). Although the isobestic point at 308 nm and hypochromism effect at 526 nm were the evidence for the formation of Ery–hsDNA complex (Fig. 2A), the acquired UV-vis spectral profiles of Ery and hsDNA highly overlapped, and the accurate information including the concentration profiles of the components (Ery, hsDNA and Ery–hsDNA complex) as well as their spectral profiles could hardly be obtained by this conventional UV-vis spectrophotometry.Considering the complicated spectral characteristics of the interaction between Ery and hsDNA, a chemometrics method, MCR-ALS was used to interpret the augmented spectroscopic data matrix [DEry, DctDNA]. As previously described in Section 2.2.1, [DEry, DctDNA] was submitted for the purpose of extracting information including the number of significant factors, f and the relative concentrations of the various reactant and product species. The extracted first four eigenvalues by singular value decomposition (SVD) method were 121.12, 60.34, 10.36 and 2.48, implying that there were three significant factors, i.e., Ery, hsDNA and Ery–hsDNA complex in the reaction system.
The concentration profiles and spectral profiles of the components (Ery, hsDNA and Ery–hsDNA complex) were extracted by MCR-ALS approach (Fig. 2C–E). A gradual decrease in Ery concentration and increase in the concentration of Ery–hsDNA complex were observed upon the addition of hsDNA (Fig. 2C). Conversely, with the addition of Ery, the concentration of hsDNA decreased, accompanied by an increase in the concentration of Ery–hsDNA complex (Fig. 2D). The extracted spectra (solid line) of Ery and hsDNA by MCR-ALS were consistent with the measured spectra (dashed line, Fig. 2E), indicating that the concentration profiles of the three reacting components were resolved correctly.32 These results provided qualitative and quantitative evidence for the interaction between Ery and hsDNA associated with the formation of the Ery–hsDNA complex.
3.2. RLS spectra characteristics of Ery–hsDNA system
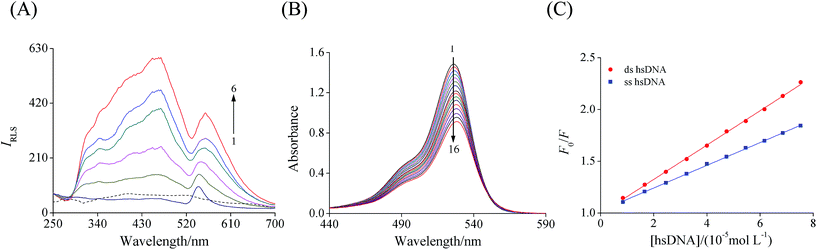
As shown in Fig. 3A, Ery (curve 1) and hsDNA (dashed curve) showed weak RLS signals under the scanning range from 250 to 700 nm. However, with an increase in the concentration of hsDNA, the RLS intensity and the peak shape of Ery were remarkably changed, accompanied by the appearance of a new peak at around 485 nm. The RLS signals are related to the formed aggregate of the particle dimension in the solution.34,35 Generally, when the diameter of a particles increases, the light scattering signal should be dramatically enhanced. Thus, it can be inferred from the results that a new Ery–hsDNA complex was formed after mixing hsDNA with Ery in solution due to an increased light scattering signal. This result further supported the result of MCR-ALS analysis.Previous studies have shown that tamoxifen36 and oridonin37 bound to ctDNA via an intercalation mode, resulting in a dramatical increase of RLS intensity. Similarly, it was reasonable to propose that the increase of the RLS signal of Ery may be due to the intercalation into hsDNA, inducing the formation of a new complex with large particle size.
3.3. UV-vis absorption spectroscopic characteristics
Due to a strong stacking interaction between the aromatic chromophore of the ligand and DNA base pairs, a large hypochromism and bathochromism or red-shift in the absorption spectra of ligand to DNA can be observed in the intercalation.38,39 However, groove and electrostatic interaction do not show these changes in absorption spectroscopic characteristics.40 Hypochromism and a significant red shift of the spectra of Ery at 526 nm were observed upon the addition of increasing concentrations of hsDNA to the Ery solution (Fig. 3B). The spectral characteristics indicated that a strong interaction in the molecular stack between the aromatic chromophore of Ery and the base pairs of hsDNA may occur due to the intercalative binding of Ery to hsDNA double-helix.38,403.4. Effects of Ery on ssDNA and dsDNA
If the binding mode is intercalation, the quenching effect of ssDNA should be weaker than that of dsDNA for the release of the double strands of DNA. By contrast, the groove mode may lead to a greater quenching effect of ssDNA than dsDNA.41 As shown in Fig. 3C, ds hsDNA exhibited a higher slope than ss hsDNA, the corresponding KSV values for ds hsDNA and ss hsDNA were determined to be 1.66 × 104 and 1.10 × 104 L mol−1, respectively. The quenching effect of ds hsDNA was greater than that of ss hsDNA, which supported the intercalative binding between Ery and hsDNA.3.5. Viscosity
Viscosity was measured to further confirm the nature of the binding between Ery and hsDNA. A classical intercalation causes the viscosity increase of DNA as the space of adjacent base pairs needs to be large enough to accommodate the bound ligand,42 while a partial or non-classical intercalation ligand could bend (or kink) the DNA helix, typically reducing its effective length and concomitantly its viscosity.43 As shown in Fig. 4, the relative viscosity of hsDNA obviously decreased with the successive addition of Ery, indicating that Ery molecules bound to hsDNA through a partial intercalation. | ||
| Fig. 4 Effect of increasing amounts of Ery on the relative viscosity of hsDNA at 298 K. c(hsDNA) = 5.12 × 10−5 mol L−1. | ||
3.6. FT-IR studies
The spectral features of free hsDNA and Ery–hsDNA complex were shown in Fig. 5A. The bands at position 1713, 1664, 1609 and 1489 cm−1 primarily corresponded to bases stretching vibrations of guanine (G), thymine (T), adenine (A) and cytosine (C), respectively. The bands located at 1222 and 1089 cm−1 are due to the asymmetric and symmetric phosphate stretching vibrations, respectively. The plots of the relative intensity (R) of several peaks of DNA in-plane vibrations related to A–T, G–C base pairs and the PO2 stretching vibrations at different Ery concentrations were obtained after peak normalization using Ri = Ii/I968, where Ii is the intensity of the absorption peak for pure DNA in the complex with i as ligand concentration, and I968 is the intensity of the 968 cm−1 peak (DNA internal reference).44 The FT-IR spectra and relative intensity of hsDNA were changed by different [Ery]/[hsDNA] ratios, which offered valuable information concerning the binding sites of Ery with hsDNA.The peak of guanine at position 1713 cm−1 shifted downward to 1702 cm−1 (by 11 cm−1) with increasing concentrations of Ery. Simultaneously, cytosine band at 1489 cm−1 was shifted upward to 1496 cm−1 by Ery–hsDNA interaction (Fig. 5A). The position changes of these bands were also accompanied by the decreases in their vibrational intensity (Fig. 5B). Compared to guanine and cytosine bands, both thymine band at 1664 cm−1 and adenine band at 1609 cm−1 as well as the asymmetric and symmetric phosphate stretching vibrations located at 1222 and 1089 cm−1 did not show significant spectral shifting. These spectral changes suggested that Ery molecules might primarily bind to the guanine and cytosine bases of hsDNA.26 Small increases in vibrational intensity of the phosphate stretching peaks (both asymmetric and symmetric) in the presence of Ery at high concentrations were observed, suggesting non-interaction between Ery and phosphate group of hsDNA backbone, but the existence of Ery may result in some perturbation to the phosphate skeleton.26
3.7. 1H NMR characteristics
Additional evidence for the interaction of Ery with hsDNA was obtained by 1H NMR study, an effective means of obtaining the binding mode of the interaction system. As shown in Fig. 6A, the 1H NMR spectrum of Ery exhibited five peaks (6.870–6.882 ppm for Hb site, 7.482–7.484 ppm for Hc site, 7.570–7.571 ppm for Hd site, 7.626 ppm for Ha sites, and 7.715–7.727 for He, Table 1). Chemical shifts of the hydrogen atoms were observed after hsDNA was added into Ery solution. The hydrogen atoms (b) and (e) moved to upfield with the maximum chemical shift values of 0.021 and 0.012, while the hydrogen atoms (c) and (d) shifted to downfield with a new splitting peak, respectively. However, the hydrogen atoms (a) did not exhibit obvious chemical shift. Evidently, the chemical shift of proton resonances of benzene ring with carboxyl sodium exhibited a significant change, indicating that the benzene ring did insert into the base pairs of hsDNA, thus there was the magnetic shielding effect in Ery–hsDNA complex due to the changes of electron density in the surroundings of the ring.45 | ||
| Fig. 6 (A) 1H NMR spectra of Ery (red) and Ery–hsDNA system (blue). (B) Molecular modeling results of the energy-minimized structure of Ery–DNA system. The green dashed lines stand for hydrogen bond. | ||
3.8. Computational modeling of Ery–hsDNA complex
As it can provide the visual representation, and even predict the probable binding characteristics of ligands to DNA, molecular docking has been widely used to investigate the binding interaction of small molecules with DNA. The molecular docking of Ery with DNA was carried out to further clarify the binding mode of Ery with DNA and the steady conformation of Ery–hsDNA complex. After the 100 docking runs were performed, the energetically most favorable conformation of the docked pose was chosen (Fig. 6B). The benzene ring part of Ery nearly vertically intercalated into hydrophobic environment of hsDNA from the small groove, and surrounded by four bases (G23, C24, G4 and G5), suggesting the existence of hydrophobic interaction and π–π interaction between Ery and hsDNA.46 Moreover, two hydrogen bonds were formed between the oxygen atoms O-1 and O-2 (Fig. 1) of Ery and the H-22 hydrogen atoms associated with N-2 of G4 and G5 of A chain, respectively. These results indicated that hydrogen bonds may play a major role in the stability of Ery–DNA structure.3.9. CD spectroscopy
CD spectroscopy is deemed as an accurate and sensitive approach to monitor the conformational change of DNA.47 As shown in Fig. 7A, the CD spectra of hsDNA exhibited a negative band at 245 nm and a positive band at 275 nm in the wavelength range of 220–320 nm, which was a signal characteristic of a typical B-DNA structure.48 The two bands are extremely sensitive in detecting DNA interaction with small molecules, and the changes of these bands often attribute to the corresponding changes in the DNA structure. The decreases in CD signals of hsDNA at both 245 and 275 nm in the presence of Ery were observed (shifting to zero level), suggesting that hsDNA structure was changed by intercalation of Ery into the base pairs of hsDNA and subsequent reduction of its base stacking and loose of the right-handed helicity.49 These results were consistent with the previous reports for other intercalators such as prodigiosin50 and perfluoroalkyl acids.513.10. DNA cleavage
Supercoiled pUC18 plasmid DNA was used to determine the DNA cleavage activity of Ery by agarose gel electrophoresis. When circular plasmid DNA is run on horizontal gel using electrophoresis, supercoiled form (Form I) will be observed in the fastest migration. While one strand or even both strands are cleaved, the supercoils will relax to become a slower-moving open circular form (Form II), and a linear form (Form III) that migrates in between Form I and Form II, respectively.52 As shown in Fig. 7B, at low Ery concentrations (0 to 1.00 × 10−4 mol L−1), Form I was only found in Lanes 1 to 5. When Ery concentration was increased (1.25 to 1.75 × 10−4 mol L−1), a mild form II was found in Lanes 6 to 8, suggesting that Ery showed a slight cleavage activity of plasmid DNA at relatively higher concentrations.3.11. Fluorescence quenching mechanism and binding constants
As shown in Fig. 8A, with increasing amounts of hsDNA, the fluorescence emission peak of Ery at 555 nm decreased gradually without apparent shift, indicating that hsDNA could interact with Ery. The fluorophore quenching results may attribute to static and dynamic quenching mechanisms. The static quenching is caused by the formation of a ground state complex, while the dynamic quenching is due to the collision between the fluorophore and the quencher.53 Static and dynamic quenching can be distinguished by their different dependence on temperature. If the quenching mechanism is static, the quenching constants would decrease with increasing temperature, while the reverse effect is observed for dynamic quenching.54The fluorescence data at different temperatures (298, 304 and 310 K) were analyzed by the Stern–Volmer plots to determine possible quenching mechanism. The plots showed a good linearity (Fig. 8B), suggesting that the predominance of only one type of quenching process occurred, either static or dynamic quenching. The KSV values for the complexation between Ery and hsDNA decreased with rising temperature (Table 2), indicating that the quenching process was primarily due to complex formation viz., static quenching.55
| T (K) | KSV (104 L mol−1) | Ra | Ka (104 L mol−1) | Rb | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) | ΔG° (kJ mol−1) | Rc |
|---|---|---|---|---|---|---|---|---|
| a Ra is the correlation coefficient for the KSV values. Rb is the correlation coefficient for the Ka values. Rc is the correlation coefficient for the ΔH° values. | ||||||||
| 298 | 1.65 | 0.9969 | 2.14 | 0.9988 | −27.65 | −9.93 | −24.69 | |
| 304 | 1.27 | 0.9993 | 1.67 | 0.9985 | −24.63 | 0.9874 | ||
| 310 | 1.01 | 0.9984 | 1.39 | 0.9983 | −24.57 | |||
The spectral data obtained from fluorescence titrations were used to construct modified Stern–Volmer plots to determine the Ka value of Ery–hsDNA (Fig. 8C). As shown in Table 2, the values of Ka at different temperatures were in the order of 104 L mol−1 and tended to decrease with increasing temperature. These results were due to reduction of the Ery–hsDNA complex stability. In addition, the values of Ka were similar to those of other intercalative additives, such as indigo carmine,10 sunset yellow,18 butylated hydroxyanisole25 and 2-tert-butyl-4-methylphenol.56
3.12. Thermodynamic parameters and binding forces
The acting forces between small molecules and macromolecules mainly include hydrogen bonds, van der Waals force, electrostatic force and hydrophobic interaction.57 The signs and magnitude of change in enthalpy (ΔH°), entropy (ΔS°) and free energy (ΔG°) for ligand–DNA interaction can be used to evaluate the main forces contributing to the formation of a ligand–DNA complex. According to the well known van't Hoff equation, the values of ΔH° and ΔS° were obtained from the slope and intercept of a linear van't Hoff plot between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka versus 1/T:
Ka versus 1/T:
 | (5) |
| ΔG° = ΔH° − TΔS° | (6) |
It is reported that when ΔH° < 0 and ΔS° > 0, the main force is electrostatic force; when ΔH° < 0 and ΔS° < 0, the main force is attributed to van der Waals and hydrogen bond, and when ΔH° > 0 and ΔS° > 0, the main force is regarded as hydrophobic interaction.58 As shown in Table 2, the values of ΔH° and ΔS° of Ery–hsDNA interaction were negative, suggesting that van der Waals and hydrogen bond played a major role in the binding of Ery to hsDNA and contributed to the stability of the complex,59 and the binding reaction was spontaneous due to the negative free energy change (ΔG° < 0).
4. Conclusions
The current work investigated the binding affinity, mode of interaction, structural aspects of the Ery–hsDNA interaction through multifaceted spectroscopy, molecular modeling and agarose gel electrophoresis. A partial intercalative binding model for Ery–hsDNA complexation was supported by the emergence of hypochromism and significant bathochromism for Ery absorption, remarkable enhancement in RLS signals of Ery upon complexation with DNA, less quenching effect by denatured hsDNA and decrease in the relative viscosity of hsDNA. The benzene ring of Ery most likely insert into the G–C bases of hsDNA and the theoretical modeling predicted and visualized the preferential docking position of Ery in hsDNA. Ery induced moderate conformational perturbations in the secondary structure of hsDNA, and this dye could lead to slight cleavage of the plasmid DNA. Moreover, the MCR-ALS algorithm was used to resolve the expanded UV-vis spectral data matrix, and the spectra of Ery, hsDNA and especially the Ery–hsDNA complex along with their concentration profiles were extracted successfully to determine the progress of Ery interaction with hsDNA. These results suggested that more attention should be paid to re-evaluate Ery as a food additive.Acknowledgements
This study is financially supported by the National Natural Science Foundation of China (No. 31460422, 21167013 and 31060210), the Joint Specialized Research Fund for the Doctoral Program of Higher Education (20123601110005), the Program of Jiangxi Provincial Department of Science and Technology (20141BBG70092), the Natural Science Foundation of Jiangxi Province (20142BAB204001 and 20143ACB20006), and the Research Program of State Key Laboratory of Food Science and Technology of Nanchang University (SKLF-ZZA-201302), which are gratefully acknowledged.References
- A. Mittal, J. Mittal, L. Kurup and A. K. Singh, J. Hazard. Mater., 2006, 138, 95–105 CrossRef CAS PubMed.
- M. Gómez, V. Arancibia, C. Rojas and E. Nagles, Int. J. Electrochem. Sci., 2012, 7, 7493–7502 Search PubMed.
- S. P. Alves, D. M. Brum, É. C. B. de Andrade and A. D. P. Netto, Food Chem., 2008, 107, 489–496 CrossRef CAS.
- B. Zhang, D. L. Du, Y. M. Yin, L. Zheng, J. H. Zhao, S. A. Eremin, V. B. Rybakov, M. Meng and R. M. Xi, Food Anal. Methods, 2014, 7, 1798–1803 CrossRef.
- F. M. D. Chequer, V. de Paula Venâncio, M. D. L. P. Bianchi and L. M. G. Antunes, Food Chem. Toxicol., 2012, 50, 3447–3451 CrossRef CAS PubMed.
- L. Pereira, B. Ali, K. Mohite, P. Arora and C. V. Rao, J. Environ. Biol., 2000, 21, 309–315 CAS.
- V. K. Gupta, A. Mittal, L. Kurup and J. Mittal, J. Colloid Interface Sci., 2006, 304, 52–57 CrossRef CAS PubMed.
- P. Mpountoukas, A. Pantazaki, E. Kostareli, P. Christodoulou, D. Kareli, S. Poliliou, C. Mourelatos, V. Lambropoulou and T. Lialiaris, Food Chem. Toxicol., 2010, 48, 2934–2944 CrossRef CAS PubMed.
- Y. F. Sasaki, S. Kawaguchi, A. Kamaya, M. Ohshita, K. Kabasawa, K. Iwama, K. Taniguchi and S. Tsuda, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2002, 519, 103–119 CrossRef CAS.
- Y. D. Ma, G. W. Zhang and J. H. Pan, J. Agric. Food Chem., 2012, 60, 10867–10875 CrossRef CAS PubMed.
- R. C. Gupta and G. Spencer–Beach, Regul. Toxicol. Pharmacol., 1996, 23, 14–21 CrossRef CAS PubMed.
- D. Sarkar, P. Das, S. Basak and N. Chattopadhyay, J. Phys. Chem. B, 2008, 112, 9243–9249 CrossRef CAS PubMed.
- S. Y. Bi, L. L. Yan, Y. Wang, B. Pang and T. J. Wang, J. Lumin., 2012, 132, 2355–2360 CrossRef CAS.
- I. Saha and G. S. Kumar, Dyes Pigm., 2013, 96, 81–91 CrossRef CAS.
- A. Tarushi, J. Kljun, I. Turel, A. A. Pantazaki, G. Psomas and D. P. Kessissoglou, New J. Chem., 2013, 37, 342–355 RSC.
- A. Basu and G. S. Kumar, J. Agric. Food Chem., 2013, 62, 317–326 CrossRef PubMed.
- N. Shahabadi and M. Maghsudi, Dyes Pigm., 2013, 96, 377–382 CrossRef CAS.
- S. Kashanian, S. H. Zeidali, K. Omidfar and N. Shahabadi, Mol. Biol. Rep., 2012, 39, 10045–10051 CrossRef CAS PubMed.
- S. Kashanian and S. H. Zeidali, DNA Cell Biol., 2011, 30, 499–505 CrossRef CAS PubMed.
- S. Ghosh, P. Kundu, B. K. Paul and N. Chattopadhyay, RSC Adv., 2014, 4, 63549–63558 RSC.
- H. Joshi, A. Sengupta, K. Gavvala and P. Hazra, RSC Adv., 2014, 4, 1015–1024 RSC.
- M. A. Husain, S. U. Rehman, H. M. Ishqi, T. Sarwar and M. Tabish, RSC Adv., 2015, 5, 64335–64345 RSC.
- M. Yang, J. Jin, G. Xu, F. Cui and H. Luo, Chem. Phys., 2014, 428, 1–5 CrossRef CAS.
- S. Kashanian, S. Javanmardi, A. Chitsazan, M. Paknejad and K. Omidfar, J. Photochem. Photobiol., B, 2012, 113, 1–6 CrossRef CAS PubMed.
- G. W. Zhang, L. H. Wang, X. Y. Zhou, Y. Li and D. Gong, J. Agric. Food Chem., 2014, 62, 991–1000 CrossRef CAS PubMed.
- Y. Zhang, G. W. Zhang, Y. Li and Y. T. Hu, J. Agric. Food Chem., 2013, 61, 2638–2647 CrossRef CAS PubMed.
- A. Nematollahi, W. B. Church, N. A. Nadvi, M. D. Gorrell and G. Sun, Cent. Nerv. Syst. Agents Med. Chem., 2014, 14, 2–9 CrossRef CAS PubMed.
- M. Garrido, F. X. Rius and M. S. Larrechi, Anal. Bioanal. Chem., 2008, 390, 2059–2066 CrossRef CAS PubMed.
- J. Jaumot, R. Gargallo, A. de Juan and R. Tauler, Chemom. Intell. Lab. Syst., 2005, 76, 101–110 CrossRef CAS.
- J. Ghasemi, S. Ahmadi, A. I. Ahmad and S. Ghobadi, Appl. Biochem. Biotechnol., 2008, 149, 9–22 CrossRef CAS PubMed.
- M. Bosco, M. P. Callao and M. S. Larrechi, Talanta, 2007, 72, 800–807 CrossRef CAS PubMed.
- Y. N. Ni, M. Wei and S. Kokot, Int. J. Biol. Macromol., 2011, 49, 622–628 CrossRef CAS PubMed.
- M. Vives, R. Gargallo and R. Tauler, Anal. Chim. Acta, 2000, 424, 105–114 CrossRef CAS.
- R. F. Pasternack, C. Bustamante, P. J. Collings, A. Giannetto and E. J. Gibbs, J. Am. Chem. Soc., 1993, 115, 5393–5399 CrossRef CAS.
- T. T. Zhao, S. Y. Bi, Y. Wang, T. J. Wang, B. Pang and T. T. Gu, Spectrochim. Acta, Part A, 2014, 132, 198–204 CrossRef CAS PubMed.
- C. Q. Cai, X. M. Chen and F. Ge, Spectrochim. Acta, Part A, 2010, 76, 202–206 CrossRef PubMed.
- Z. G. Chen, Z. Wang, J. H. Chen, X. Chen, J. H. Wu, Y. Y. Wu and J. Y. Liang, Eur. J. Med. Chem., 2013, 66, 380–387 CrossRef CAS PubMed.
- P. Paul and G. S. Kumar, Spectrochim. Acta, Part A, 2013, 107, 303–310 CrossRef CAS PubMed.
- H. Chao, W. J. Mei, Q. W. Huang and L. N. Ji, J. Inorg. Biochem., 2002, 92, 165–170 CrossRef CAS PubMed.
- M. Sirajuddin, S. Ali and A. Badshah, J. Photochem. Photobiol., B, 2013, 124, 1–19 CrossRef CAS PubMed.
- S. Kashanian, M. M. Khodaei, H. Roshanfekr, N. Shahabadi and G. Mansouri, DNA binding, Spectrochim. Acta, Part A, 2012, 86, 351–359 CrossRef CAS PubMed.
- N. Shahabadi, N. Fatahi, M. Mahdavi, Z. K. Nejad and M. Poirfoulad, Spectrochim. Acta, Part A, 2011, 83, 420–424 CrossRef CAS PubMed.
- J. H. Shi, J. Chen, J. Wang and Y. Y. Zhu, Spectrochim. Acta, Part A, 2015, 136, 443–450 CrossRef CAS PubMed.
- D. K. Jangir, G. Tyagi, R. Mehrotra and S. Kundu, J. Mol. Struct., 2010, 969, 126–129 CrossRef CAS.
- F. Wang, J. H. Yang, X. Wu, F. Wang and H. H. Ding, Spectrochim. Acta, Part A, 2007, 67, 385–390 CrossRef PubMed.
- P. Deepa, P. Kolandaivel and K. Senthilkumar, Mater. Sci. Eng., C, 2012, 32, 423–431 CrossRef CAS.
- N. Shahabadi, M. Falsafi and N. H. Moghadam, J. Photochem. Photobiol., B, 2013, 122, 45–51 CrossRef CAS PubMed.
- A. Subastri, C. H. Ramamurthy, A. Suyavaran, R. Mareeswaran, P. L. Rao, M. Harikrishna, M. S. Kumar, V. Sujatha and C. Thirunavukkarasu, Int. J. Biol. Macromol., 2015, 78, 122–129 CrossRef CAS PubMed.
- X. Y. Xu, D. D. Wang, X. J. Sun, S. Y. Zeng, L. W. Li and D. Z. Sun, Thermochim. Acta, 2009, 493, 30–36 CrossRef CAS.
- L. Han, Y. H. Zhou, X. Q. Huang, M. S. Xiao, L. Zhou, J. H. Zhou, A. H. Wang and J. Shen, Spectrochim. Acta, Part A, 2014, 123, 497–502 CrossRef CAS PubMed.
- J. Cao, Y. Wei and Y. Cheng, Environ. Sci. Pollut. Res. Int., 2013, 20, 8355–8363 CrossRef CAS PubMed.
- G. W. Zhang, L. H. Wang, X. Y. Zhou, Y. Li and D. Gong, J. Agric. Food Chem., 2014, 62, 991–1000 CrossRef CAS PubMed.
- S. R. Feroz, S. B. Mohamad, N. Bujang, S. N. A. Malek and S. Tayyab, J. Agric. Food Chem., 2012, 60, 5899–5908 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer Publications, New York, 3rd edn, 2006 Search PubMed.
- Y. Sameena and I. V. Enoch, J. Lumin., 2013, 138, 105–116 CrossRef CAS.
- S. Kashanian and J. E. N. Dolatabadi, Eur. Food Res. Technol., 2010, 230, 821–825 CrossRef CAS.
- T. Sarwar, M. A. Husain, S. U. Rehman, H. M. Ishqi and M. Tabish, Mol. BioSyst., 2015, 11, 522–531 RSC.
- J. L. Yuan, H. Liu, X. Kang, Z. Lv and G. L. Zou, J. Mol. Struct., 2008, 891, 333–339 CrossRef CAS.
- P. D. Ross and S. Subramanian, Biochemistry, 1981, 20, 3096–3102 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |