Formation mechanism of spinel LiTi2O4 prepared by carbon thermal reduction reaction†
Guijun Yangab,
Jianwen Yang*ac and
Lingzhi Zhang*b
aCollege of Chemistry and Bioengineering, Guilin University of Technology, Guilin 541004, Guangxi, China. E-mail: yangjw@glite.edu.cn; Fax: +86 773 5896839; Tel: +86 773 2538354
bKey Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, Guangdong, China. E-mail: lzzhang@ms.giec.ac.cn; Fax: +86 20 37246026; Tel: +86 20 37246025
cGuangxi Key Laboratory of Electrochemical and Magneto-chemical Functional Materials, Guilin University of Technology, Guilin 541004, China
First published on 9th November 2015
Abstract
The formation mechanism of LiTi2O4, prepared by a carbon thermal reduction reaction using Li2CO3 and TiO2 (anatase) as starting materials and acetylene black as a reducing agent, was investigated by in situ variable temperature X-ray diffraction and thermal gravimetric analysis/differential scanning calorimetry system. It was found that the cooling rate significantly impacts on obtaining pure phase LiTi2O4 sample after forming LiTi2O4 product during the carbon thermal reduction reaction. LiTi2O4 has excellent cycling stability, remaining a specific capacity of 126.6/111.9 mA h g−1 with a capacity fade of 5.1%/3.1% at 0.5C/1C rate after 200 cycles.
In recent years, great efforts have been made to develop spinel-structured Li4Ti5O12 as an alternative replacement for currently used graphite anode material in lithium-ion batteries (LIBs) due to its superior advantages such as high power capability, unique safety performance (1.55 V vs. Li/Li+), and excellent cycling stability (zero-strain insertion material).1,2 But Li4Ti5O12 has a poor electrical conductivity (<10−13 S cm−1) which seriously limits its high-rate performances for practical applications.3 Spinel LiTi2O4 has a similar crystal structure and lithium-ion migration path with Li4Ti5O12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and especially has a significantly higher electrical conductivity of 5.56 × 106 S cm−1
4 and especially has a significantly higher electrical conductivity of 5.56 × 106 S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–7 compared with Li4Ti5O12. These features make LiTi2O4 a more promising anode material as a replacement of Li4Ti5O12 in lithium-ion batteries.
5–7 compared with Li4Ti5O12. These features make LiTi2O4 a more promising anode material as a replacement of Li4Ti5O12 in lithium-ion batteries.
Various approaches have been explored to synthesize LiTi2O4, such as solid state reaction,8,9 sol–gel method,10,11 molten salts electrolysis,12,13 hydrothermal reaction.14 Among of these methods, solid-state reaction usually uses titanium5 or low-valence titanium oxides15 as reductants or other reducing elements such as lithium or hydrogen.16 However, most synthesis reported requires rigorous conditions, costly starting materials or dangerous hydrogen gas. Moreover, the synthesis of spinel LiTi2O4 with pure phase is difficult compared to Li4Ti5O12, because of the existence of mixed valence state titanium ions (Ti3+ and Ti4+) in LiTi2O4. In 2010, we reported an improved one-step carbon thermal reduction method to prepare LiTi2O4 using Li2CO3 and anatase as starting materials and acetylene black as a reducing agent.17 Nonetheless, we have reproducible problem to prepare spinel LiTi2O4 with pure phase due to the unclear underlying mechanism during the solid state reaction.
In this work, we investigate the formation mechanism of LiTi2O4 in our one-step carbon thermal reduction reaction using in situ variable temperature X-ray diffraction (VT-XRD) and thermal gravimetric analysis/differential scanning calorimetry system (TGA-DSC). The electrochemical performances of pure spinel LiTi2O4 are also reported.
In situ VT-XRD was carried out on a PANalytical X'Pert Powder diffractometer with Cu Kα radiation (λ = 1.5405 Å), equipped with an Anton Parr HTK 1200N high temperature attachment. The samples were heated in N2 atmosphere from room temperature to 900 °C with a heating rate of 10 °C min−1, and then stabilized for 30 min at each integer point of temperature. SDTQ600 thermal gravimetric analysis/differential scanning calorimetry system was used to identify the phase transition temperature in N2 atmosphere. The morphologies of the LiTi2O4 samples at different cooling rate were observed by SEM. 7Li MAS NMR spectrum of LiTi2O4 was acquired on an AV 400 Bruker spectrometer under magic angle sinning at 5 kHz using 4 mm zirconium rotors. CV was conducted in cells at 0.2 mV s−1 from 0.8 V to 2.5 V. EIS was measured by applying an alternating voltage of 5 mV over the frequency ranging from 102 to 105 Hz.
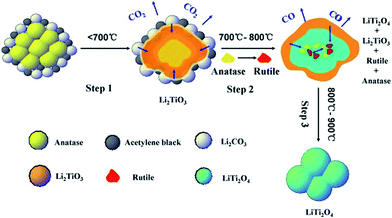
The in situ VT-XRD measurement was used to analyze the formation mechanism of LiTi2O4 during the carbon thermal reduction. The XRD patterns over heating from room temperature to 900 °C in a 2θ range of 10°–70° are shown in Fig. 1a. The XRD patterns of the starting materials before heating are well consisted with the corresponding crystal structure in the database. The peaks at 20.3°, 35.8° and 43.4° corresponding to (0 2 0), (−1 3 1) and (2 0 2) plane of Li2TiO3 intermediate started to appear at 600 °C. At 700 °C, the peaks at 35.5° and 40.3° corresponding to (3 1 1) and (4 0 0) plane of LiTi2O4 appeared, suggesting that LiTi2O4 product was generated since 700 °C. The peak at 18° corresponding to (1 1 1) plane of LiTi2O4 and Li2TiO3 was not distinguishable due to overlapping of these two phases. After heating to 800 °C and kept for 30 min, anatase disappeared completely. The intensity of rutile increased rapidly because of anatase turned into rutile from 700 °C to 800 °C, and then decreased after 800 °C due to its continuous consumption by reacting with Li2TiO3 to generate LiTi2O4. In our experiments, rutile completely disappeared when the temperature was heated to 900 °C and kept for 12 h.
It worth to point out that the cooling rate has a significant impact on obtaining pure phase LiTi2O4 sample.18 Fig. 1b shows the in situ VT-XRD patterns of LiTi2O4 product cooling from 900 °C to room temperature with a rate of 10 °C min−1. As the temperature decreased after 850 °C, the diffraction peaks of LiTi2O4 product shifted to larger angles, indicating that the impurity was generated in the product (Fig. 1b). A comparative experiment was conducted by cooling the LiTi2O4 product at different rate of 180 °C min−1 and 10 °C min−1, respectively. At a fast cooling rate of 180 °C min−1, the pure phase LiTi2O4 was obtained as a deep-blue powder with well-defined and sharp XRD diffraction peaks which is consistent well with the reference LiTi2O4 (JCPDS no. 82-2318, Fig. 1c). As comparison, impurity was observed after cooling at 10 °C min−1 as shown in Fig. 1c.
SEM observation shows that LiTi2O4 sample cooled at 180 °C min−1 has a size range of 400 to 500 nm with perfect cubic shape (Fig. S1a†). As comparison, LiTi2O4 sample cooled at 10 °C min−1 has similar size, but consists of a lot of shapeless broken particles (Fig. S1b†). The 7Li MAS NMR spectrum of pure LiTi2O4 cooled at 180 °C min−1 displays only one chemical shift at −0.053 ppm with three symmetric spinning sidebands at 42.957/−43.082, 85.727/−85.775, 128.510/−128.531 ppm (Fig. S1c†), due to the average oxidation state of Ti3+ and Ti4+ ions in LiTi2O4.18,19
Based on the above results, the formation mechanism of LiTi2O4 was proposed as following, and schematically illustrated in Scheme 1:
 | (1) |
 | (2) |
 | (3) |
 | (4) |
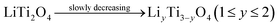 | (5) |
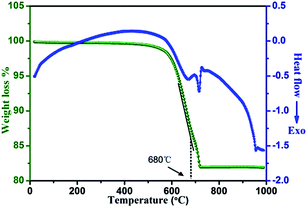
TGA-DSC experiment was carried out from room temperature to 1000 °C to further confirm the formation mechanism of LiTi2O4 during the solid state reaction (Fig. 2). The TGA curve of the reaction mixture (Li2CO3, TiO2 and acetylene black) for synthesizing LiTi2O4 showed a weight loss of 0.83% at a temperature range of room temperature to 500 °C due to the dehydration of the reactants which is correlated with the broad endothermic peak at the same temperature range in DSC curve. The second weight loss of 18.3% at 500 °C–720 °C in TGA curve corresponds to the exothermic peak at the same temperature range in DSC curve which composes of two relatively sharp exothermic peaks at 580 °C–680 °C and 680 °C–720 °C, respectively. These two sharp exothermic peaks correspond to the eqn (1) reaction with a weight loss of 11.61 wt% (fitting well with the theoretical value of 10.83 wt%) and the eqn (2) reaction between Li2TiO3 and anatase with a weight loss of 6.69 wt% (fitting well with the theoretical value of 7.01 wt%), respectively.
To correlate with the previous XRD data, the intensities of in situ VT-XRD peaks for reactants, intermediate and product are normalized as shown in Fig. 3. The small sharp exothermic peak observed in Fig. 2 from 720 °C to 750 °C corresponds to the phase transformation of anatase to thermodynamically stable rutile in eqn (3).20 Anatase disappeared completely at 800 °C, evidenced by that the normalized intensity of anatase decreased to zero (800-2C in Fig. 3a). It is worth to note that Li2TiO3 continued to react with rutile to generate LiTi2O4 product after 800 °C (eqn (4)) which correlates well with the intensity change of rutile, Li2TiO3 and LiTi2O4 product (Fig. 3b–d). There is no obvious weight loss in TGA curve, mainly because of the very little amount of the rutile and the reaction is slow. Therefore, it is necessary to keep at 900 °C for 12 h in experiments to obtain the LiTi2O4 with pure phase.
 | ||
| Fig. 3 The normalized intensities of in situ VT-XRD peaks for (a) anatase, Li2CO3 (b) intermediate Li2TiO3 and (c) LiTi2O4 product and (d) rutile at the heating and cooling temperature. | ||
CV and EIS measurements were used to characterize and compare the electrochemical kinetics of LiTi2O4 electrode at different cooling rates. Both samples display a pair of reversible oxidation and reduction peaks at 1.63/1.44 V and 1.86/1.33 V for LiTi2O4 cooled at 180/10 °C min−1, respectively (Fig. S2a†). But LiTi2O4 cooled at 180 °C min−1 shows relatively sharper peaks and smaller potential difference (ϕa − ϕb) of 0.19 V between anodic and cathodic peaks as compared with 0.53 V for LiTi2O4 cooled at 10 °C min−1, suggesting a lower polarization of the electrode. The Nyquist plot for LiTi2O4 electrode at different cooling rate both consists of a semicircle and a linear part (Fig. S2b†); the fitted parameters including the electrolyte resistance (Rs), the charge-transfer resistance (Rct), the double layer capacitance and passivation film capacitance (CPE), the exchange current density (i0 = RT/nFRct) are collected in Table S1.†21 These data, together with the above CV results, imply that LiTi2O4 sample cooled at 180 °C min−1 has an improved electrical conductivity and better electrochemical kinetics as compared with LiTi2O4 sample cooled at 10 °C min−1.
The voltage–capacity curve for the initial charge/discharge cycle at 0.2C is shown as the inset in Fig. 4a. The potential profile of LiTi2O4 electrode exhibited a typical long and flat potential plateau at around 1.55 V (vs. Li/Li+), corresponding to the lithium insertion/extraction reaction between LiTi2O4 and Li2Ti2O4 phase. At 0.2C rate, the electrode showed a reversible discharge capacity of 144.5 mA h g−1 with a coulombic efficiency of 98% between 1.0 and 3.0 V. The long-term cycling performances of LiTi2O4 electrode at 0.5C and 1C rate are displayed in Fig. 4a. The LiTi2O4 electrode showed an initial charge capacity of 133.4/115.1 mA h g−1 at 0.5C/1C rate, respectively. Over 200 cycles, LiTi2O4 electrode remarkably retained a stable cycling capacity of 126.6/111.9 mA h g−1 with corresponding capacity retention of 94.9%/96.9% at 0.5C/1C rate, respectively. These data are better, in terms of capacity and cycling stability, than those of spinel LiTi2O4 samples recently reported in literature,22,23 even including that doped with metal ions (Fe and V).24,25 Fig. 4b shows the rate capability of the LiTi2O4 electrode. The current density was increased gradually from 0.2C to 10C and then returned back to 0.2C. When the rate increased from 0.2C to 5C, the LiTi2O4 electrode maintained an average reversible capacity of 54.3 mA h g−1. When the current density was returned back to 0.2C, a stable reversible capacity of 137.5 mA h g−1 was obtained, which is about 95.2% of the initial charge capacity at 0.2C, indicating that the integrity of LiTi2O4 electrode was maintained even after high rate charge and discharge tests.
 | ||
| Fig. 4 (a) The cycling performance of LiTi2O4 during 180 °C min−1 cooling at 0.5C/1C rate and (b) rate capability. | ||
Conclusions
The formation mechanism of LiTi2O4 in the one-step solid-state reaction using Li2CO3 and TiO2 (anatase) as starting materials and acetylene black as reductant is studied by in situ VT-XRD measurements and TGA-DSC analysis. The formation of spinel LiTi2O4 consists of three steps. Firstly, Li2CO3 reacts with TiO2 (anatase) to form intermediate Li2TiO3. Secondly, Li2TiO3 reacts with TiO2 (anatase) in the presence of acetylene black to form LiTi2O4 at 700 °C. In the same time, anatase starts to transform into rutile at 700 °C. Thirdly, Li2TiO3 continues to react with TiO2 (anatase and rutile) and acetylene black to generate LiTi2O4 product. Interestingly, the cooling rate significantly impacts on obtaining pure phase LiTi2O4 sample. Pure LiTi2O4 sample can be reproducibly prepared as a deep-blue powder at a fast cooling rate of 180 °C min−1. Comparative CV and EIS study shows that LiTi2O4 cooled at 180 °C min−1 has improved electrical conductivity and favorable electrochemical kinetics as compared with the sample cooled at 10 °C min−1. The electrochemical investigation shows that LiTi2O4 exhibits excellent cycling stability with a capacity retention of 96.9% at 1C after 200 cycles. We believe that this work will construct a solid foundation for optimizing the synthesis and further developing LiTi2O4 as anode material for lithium-ion batteries.Acknowledgements
This work was supported by National Natural Science Foundation of China (51164006/21573239), CAS Key Laboratory of Renewable Energy (Y407K41001), Science & Technology Program of Guangxi Province (1298025-9), Special Support Program of Guangdong Province for High-Level Talents (2014TX01N014), Collaboration Project of CAS-Guangdong Province (2013B09 1300017) and Guangzhou Municipal Project for Science & Technology (201423/2014Y2-00219).Notes and references
- T. Ohzuku, A. Ueda and N. Yamamoto, J. Electrochem. Soc., 1995, 142, 1431–1435 CrossRef CAS.
- G. Q. Zhang, B. Y. Xia, C. Xiao, L. Yu, X. Wang, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 8643–8647 CrossRef CAS PubMed.
- Q. Y. Zhang, H. S. Lu, H. X. Zhong, X. D. Yan, C. Y. Ouyang and L. Z. Zhang, J. Mater. Chem. A, 2015, 3, 13706–13716 CAS.
- T. F. Yi, Y. Xie and Y. R. Zhu, J. Power Sources, 2012, 198, 318–321 CrossRef CAS.
- K. M. Colbow, J. R. Dahn and R. R. Haering, J. Power Sources, 1989, 26, 397–402 CrossRef CAS.
- A. Kuhn, C. Baehtz and F. Garcia-Alvarado, J. Power Sources, 2007, 174, 421–427 CrossRef CAS.
- H. X. Geng, A. F. Dong, G. C. Che, W. W. Huang, S. L. Jia and Z. X. Zhao, Physica C, 2005, 432, 53–58 CrossRef CAS.
- M. Manickam and M. Takata, J. Power Sources, 2003, 114, 298–302 CrossRef CAS.
- J. A. Mergos and C. T. Dervos, Mater. Charact., 2009, 60, 848–857 CrossRef CAS.
- L. Persi, F. Croce and B. Scrosati, Electrochem. Commun., 2002, 4, 92–95 CrossRef CAS.
- M. Łapiński, B. Kościelska and W. Sadowski, J. Phys. Chem. Solids, 2013, 74, 575–578 CrossRef.
- N. Kumada, M. H. K. Rubel, A. Miura and T. Takei, J. Ceram. Soc., 2014, 122, 307–309 CrossRef.
- J. Akimoto, Y. Gotoh, K. Kawaguchi and Y. Oosawa, J. Solid State Chem., 1992, 96, 446–450 CrossRef CAS.
- D. Fattakhova, V. Petrykin, J. Brus, T. Kostlánová, J. Dědeček and P. Krtil, Solid State Ionics, 2005, 176, 1877–1885 CrossRef CAS.
- A. Kuhn, M. Martín and F. García-Alvarado, J. Solid State Chem., 2010, 183, 20–26 CrossRef CAS.
- T. Kanno, J. Awaka, F. Kariya, S. Ebisu and S. Nagata, Phys. B, 2006, 381, 30–33 CrossRef CAS.
- J. W. Yang, J. Zhao, Y. Z. Chen and Y. Li, Ionics, 2010, 16, 425–429 CrossRef CAS.
- J. P. Kartha, D. P. Tunstall and J. T. S. Irvine, J. Solid State Chem., 2000, 152, 397–402 CrossRef CAS.
- C. P. Grey and N. Dupre, Chem. Rev., 2004, 104, 4493–4512 CrossRef CAS PubMed.
- K. J. D. Mackenzie and P. J. Melling, Trans. J. Br. Ceram. Soc., 1974, 73, 179–183 CAS.
- L. Yue, S. Q. Wang, X. Y. Zhao and L. Z. Zhang, J. Mater. Chem., 2012, 22, 1094–1099 RSC.
- C. Q. Feng, L. Li and Z. P. Guo, J. Alloys Compd., 2009, 478, 767–770 CrossRef CAS.
- M. J. Pan, Y. X. Chen and H. B. Liu, Ionics, 2015, 21, 2417–2422 CrossRef CAS.
- S. Chakrabarti, A. K. Thakur and K. Biswas, Solid State Ionics, 2014, 262, 49–55 CrossRef CAS.
- J. Liu, C. Q. Du, Y. Q. Lin, Z. Y. Tang and X. H. Zhang, J. Alloys Compd., 2015, 622, 250–253 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the synthesis, characterization, and electrochemical measurements. See DOI: 10.1039/c5ra19621c |
| This journal is © The Royal Society of Chemistry 2015 |