Studies of dispersed liquid crystals in binary mixtures with ionic liquid and their excitation by electric signals
Abstract
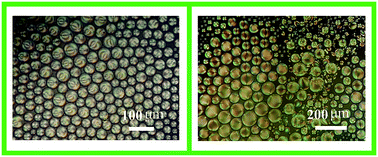
We report the results of dispersion studies of two nematogens (i) 6CHBT (4-(trans-4-n-hexylcyclohexyl)isothiocyanatobenzene) and (ii) PCH5 (4-(trans-4-pentyl-cyclohexyl)benzonitrile) in their binary mixtures with phosphonium based ionic liquid [P6,6,6,14][Ntf2] (trihexyl(tetradecyl)phosphoniumbis(trifluoromethylsulfonyl)amide). The morphology and properties of these mixtures have been investigated by using differential scanning calorimetry (DSC), polarizing optical microscopy (POM), Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and, their excitations by electric signals. For both the liquid crystals, nematic droplets are found to be dispersed in the ionic liquid having a particular anion, i.e., [Ntf2]. These droplets closely resemble the common droplet morphologies observed in polymer dispersed liquid crystals. Thermal and morphological investigations on the mixtures show a decrease in the peak value of the isotropic to nematic transition and broadening of the associated peak with a decrease in the compositions of nematogens in mixtures. As a result, nematic droplets in a wide temperature range ∼20 °C (i.e., from ∼16 °C to ∼36 °C) are observed for various mixtures. Electrical excitations of the mixtures show significant changes in the droplet size and nematic director orientation inside the droplets due to change in the anchoring conditions with the application of external electric pulses. The influence of the electric field on these nematic droplets and their stability over a wide temperature range suggests their potential for various device development applications.

 Please wait while we load your content...
Please wait while we load your content...