Construction of 3,5-dinitrated 1,4-dihydropyridines modifiable at 1,4-positions by a reaction of β-formyl-β-nitroenamines with aldehydes†
Haruyasu Asahara*ab,
Mai Hamadaa,
Yumi Nakaike‡
a and
Nagatoshi Nishiwaki*ab
aSchool of Environmental Science and Engineering, Kochi University of Technology, Kami, Kochi 782-8502, Japan. E-mail: nishiwaki.nagatoshi@kochi-tech.ac.jp; asahara.haruyasu@kochi-tech.ac.jp; Fax: +81-887-57-2520; Tel: +81-887-57-2517
bResearch Center for Material Science and Engineering, Kochi University of Technology, Kami, Kochi 782-8502, Japan
First published on 16th October 2015
Abstract
A novel and efficient method for the synthesis of 4-substituted 3,5-dinitro-1,4-dihydropyridines by a reaction of β-formyl-β-nitroenamines with aldehydes was developed. The reaction of nitroenamines with aldehydes leading to 1,4-dihydropyridines and the self-condensation of nitroenamines leading to pyridinium salt intermediate proceed competitively. The obtained 3,4,5-trisubstituted-1,4-dihydropyridines readily transformed into the corresponding pyridines in high yields.
Introduction
1,4-Dihydropyridine (DHP) derivatives have attracted great attention in the medicinal chemistry and pharmacological fields due to their wide spectrum of bioactivities.1 4-Arylated DHPs are often found as the fundamental framework in drugs such as calcium antagonists2 and also the drugs for cardiovascular diseases.3 Moreover, dimeric DHPs are used as precursors for HIV-1 protease inhibitors.4 Apart from their medicinal value, DHPs, especially 2,6-unsubstituted DHPs with electron-withdrawing groups at the 3- and 5-positions, have been employed as photoelectronic functional materials.5 Thus, numerous methods for the preparation of DHPs have been reported. The Hantzsch reaction, a multi-component reaction of an aldehyde, two β-keto esters, and ammonia, is the most widely used.6 However, only a few cases of the synthesis of 2,6-unsubstituted 3,5-functionalized DHPs have been reported so far; in particular, the introduction of nitro groups at the 3- and 5-positions is quite difficult, mainly because of poor reactivity.Considering this background, we recently developed a novel method for the construction of 4-arylated 3,5-dinitro-1,4-dihydropyridines (dinitro-DHPs) (Scheme 1(a))7 by electrophilic substitution of electron-rich benzene derivatives with pyridinium ion X, formed by self-condensation of β-formyl-β-nitroenamine 1. The formylnitroenamine 1 is useful synthetic unit because its versatile reactivity arises from two electrophilic sites, nucleophilic amino group, and electron-withdrawing nitro group. It is easily prepared from commercially available reagents by a few steps, and they are easily handled because of the high solubility in common organic solvents. Although this reaction affords dinitro-DHPs that are not easily formed by other methods, the substrate scope is limited to highly electron-rich aromatics. Thus, the development of an efficient method for the synthesis of dinitro-DHPs having various groups is highly desirable.
Based on the Hantzsch DHP synthesis,8 which includes a protonated α,β-unsaturated ketone and an enamine as key intermediates (Scheme 1(b)), a multi-component reaction between two molecules of β-formyl-β-nitroenamine 1 and an aldehyde 2 is designed (Scheme 1(c)). In this strategy, the nitroenamine serves as both an α,β-unsaturated iminium and an enamine, enhancing the synthetic utility of formylnitroenamine as a building block. Herein, we report a new method for the construction of various 4-substituted 3,5-dinitro-1,4-DHPs 3 and their oxidation to afford 3,5-dinitropyridines.
Results and discussion
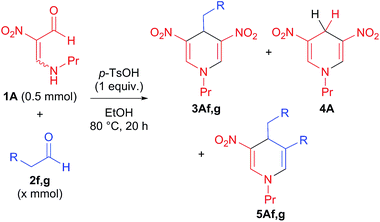
At first, we studied the reaction of N-propyl-β-formyl-β-nitroenamine 1A (0.5 mmol) with p-tolualdehyde (Tol-CHO) 2a under acidic conditions (Table 1). When the reaction was carried out in the presence of p-toluenesulfonic acid monohydrate (p-TsOH·H2O, 0.25 mmol) in ethanol, the desired 4-arylated dinitro-DHP 3Aa was successfully obtained in 67% yield (entry 1, based on half of 1A), accompanied by the formation of 4-unsubstituted DHP 4A (4%). Although the yield of the desired product 3Aa slightly increased up to 78% when 0.5 mmol of p-TsOH was used, the yield of the by-product 4A also increased (entry 2). However, higher amounts of p-TsOH did not significantly affect the reaction (entry 3, for additional optimizations see ESI, Table S1†). As depicted in Scheme 2, the 4-unsubstituted DHP 4A was formed by the reduction of the pyridinium ion X, which is obtained by self-condensation of 1A in the absence of aldehyde 2 (more details on the reduction of X by alcohol are discussed later). From this viewpoint, increasing the amount of aldehyde was assumed to be effective for suppressing the competitive formation of the by-product 4A. Accordingly, when 2.5 mmol of 2a was used, the desired product 3Aa was successfully isolated in 90% yield with negligible amounts of 4A (entry 5).With the optimal conditions being derived, the scope and limitation of this reaction were examined using various aromatic aldehydes 2b–e (Table 2). In all cases, the desired 4-arylated dinitro-DHPs 3 were obtained in high yields. It is noteworthy that, unlike previous methodologies, electron-neutral (entry 2) and electron-deficient aromatic rings (entries 3 and 4) could be introduced.
Next, we examined the substrate scope using a more reactive aliphatic aldehyde, i.e., butanal 2f (Table 3). Although the desired product 3Af was obtained, the yield was lower than that of 4-arylated DHPs 3Aa–e, and the 3,4-dialkyl-DHP derivative 5 was also formed (entry 1). Product 5 formed by the Michael addition of the nitroenamine 1A to the α,β-unsaturated enone that is formed by aldol condensation of butanal 2f (Scheme 3). This competitive reaction was suppressed by decreasing the amount of aldehyde 2f, thereby increasing the yield of dinitro-DHP 3Af up to 45%, accompanied by 19% of unsubstituted product 4A (entry 2). The undesired aldol reaction of the aldehyde was suppressed by using the bulkier 3-methylbutanal 2g, which successfully afforded dinitro-DHP 3Ag in 70% isolated yield (entry 3).
In order to facilitate the modification at the 1-position of the dinitro-DHPs, application of the procedure optimized for 1A to N-tert-butyl-β-formyl-β-nitroenamine 1B was attempted. Nitroenamine 1B reacted similarly with aromatic aldehydes 2a–e to afford the corresponding 4-arylated dinitro-DHPs 3Ba–e in good yields (Table 4, entries 1–5, Fig. S1†), whereas the reaction with aliphatic aldehydes 2f and 2g gave 3Bf and 3Bg in low yields (entries 6 and 7). It is noteworthy that, in all cases, 3,5-dinitropyridine 6 was obtained in moderate yields. A plausible mechanism for the formation of 6 is shown in Scheme 2. Upon treatment of 1B with acid, two molecules of 1B underwent a formal [4 + 2] condensation to form the pyridinium ion intermediate XB, from which the stable tert-butyl cation was eliminated to afford the 3,5-dinitropyridine 6. As expected, 6 was produced in 70% yield when 1B was treated under the same conditions in the absence of aldehyde 2 (entry 8). The highly electron-deficient heteroarene 6 is a potentially useful and versatile synthetic intermediate;9 however, its conventionally used preparation method suffers from troublesome multi-step reactions and low total yield.10 Thus, our reaction using N-tert-butyl-β-formyl-β-nitroenamine 1B represents an alternative method for the rapid construction of 3,5-dinitropyridine 6 in high yield.
In this method, in addition to 4-substituted DHPs 3, 4-unsubstituted DHPs 4 were obtained as by-products. In order to gain an insight into the mechanism of the formation of 4-unsubstituted DHPs 4, several control experiments were conducted (Scheme 4). When 1A was treated with TsOH in CD3CD2OD (ethanol-d6), 4-monodeuterated 3,5-dinitro-DHP was obtained in 55% yield along with 10% of 4-CD3-3,5-dinitro-DHP (Scheme 4(a)). Moreover, when benzyl alcohol was employed as solvent instead of ethanol, 4-phenyl-3,5-dinitro-DHP 3Ac and 3,5-dinitro-DHP 4 were obtained in 33% and 66% yields, respectively (Scheme 4(b)). In addition, the formation of benzaldehyde was confirmed by the 1H NMR spectrum of the reaction mixture. These observations obviously indicated that the pyridinium ion X was reduced by the alcohol via formation of the 4-position alcohol adduct intermediate Y (Scheme 5).
Subsequently, the transfer of the R group leads to 4-substituted products 3 (route a), whereas the intramolecular hydride transfer gives the reduced product 4 (route b). Herein, the reduction of pyridinium ion by alcohol is often found in the biological system, such as NAD+/NADH system in the presence of dehydrogenase.11 On the other hand, Lu et al. thoroughly studied the mechanism of the reduction of pyridinium ion derivatives by an alcohol in the presence of Brønsted acid and showed the reaction involves intermolecular hydride transfer as transition state similar to biological system.12 Contrary to these examples, our reaction is first example for the reduction of pyridinium salt by alcohol via intramolecular hydride or alkyl transfer mechanism.
With 4-arylated dinitro-DHPs in hand, we carried out preliminary studies on the oxidative conversion of 4-substituted DHPs to 4-substituted-3,5-dinitropyridines, which are not easily accessible by other methods.13 As shown in Scheme 6, treatment of 4-anisyl DHP 3Bb with excess amount of NaNO2 in chloroform under oxygen atmosphere at 80 °C for 24 h to afford the desired product 7 in a promising isolated yield of 68%. The obtained 4-arylated-3,5-dinitropyridines are useful synthetic intermediates for functional materials. Moreover, the push–pull electronic properties of this product are crucial for developing organic materials with potential applications in nonlinear optics.
Conclusions
In conclusion, we have successfully developed a multi-component reaction of β-formyl-β-nitroenamines 1 and aldehydes 2 leading to the formation of diverse 4-substituted 3,5-dinitro-DHPs. This method provides an environmentally benign and metal-free access to a variety of 2,6-unsubstituted 3,5-dinitro-DHPs, which have not been extensively studied because of the synthetic difficulties. From a mechanistic point of view, competitively formed self-condensed pyridinium ion X showed interesting reactivity. Namely, alcohols regioselectively attack to 4-position of intermediate X to form adduct intermediate Y, and then is oxidized via intramolecular hydride or alkyl transfer. This is first example for the reduction of pyridinium salt by alcohol via intramolecular process. In addition, the synthesized DHPs are easily transformed to 4-substituted 3,5-dinitropyridines, which have high potential for nonlinear optical materials. Further applications of the electronic properties of the obtained products in this work are currently underway.Experimental
General procedure for the reaction of N-propyl-β-formyl-β-nitroenamines 1A with p-tolualdehyde 2a
p-TsOH·H2O (95 mg, 0.5 mmol) was added to the suspension of β-formyl-β-nitroenamine 1A (0.5 mmol) and p-tolualdehyde 2a (306 μL, 2.5 mmol) in ethanol (1 mL), and the resultant mixture was stirred at 80 °C for 20 h in a sealed tube. The solvent was then evaporated in vacuo, and the residue was diluted with CH2Cl2 (20 mL) and washed with water (10 mL × 3). After drying (MgSO4) and evaporation of the solvent, the residue was purified on silica gel column chromatography (hexane/ethyl acetate = 5/1). Further purification was performed by recrystallization from chloroform. All reactions of nitroenamines with aldehydes were conducted according to general procedure. Compound 3Ab is known and its spectral data match with reported data.7General procedure for the synthesis of 4-arylated-3,5-dinitropyridine 7
The oxidation was performed according to literature procedure.13 To a suspension of 3,5-dinitro-DHP 3Bb (0.3 mmol, 83 mg) in acetic acid (3 mL) and chloroform (0.5 mL), sodium nitrite (1.5 mmol) was added. The mixture was stirred under oxygen at 80 °C for 24 h in a sealed tube. The mixture was then evaporated, and the residue was diluted with CH2Cl2 (20 mL) and washed with water (10 mL × 3). After drying (MgSO4) and evaporation of the solvent, the residue was purified by column chromatography on silica gel (hexane/ethyl acetate = 9/1).1,4-Dihydro-3,5-dinitro-4-(4-methylphenyl)-1-propylpyridine (3Aa)
Orange solid (71.9 mg, 90%): mp 182–184 °C; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 1.07 (t, J = 7.2 Hz, 3H), 1.85 (tq, J = 7.2, 7.2 Hz, 2H), 2.29 (s, 3H), 3.57 (t, J = 7.2 Hz, 2H) 5.61 (s, 1H), 7.10 (d, J = 8.4 Hz, 2H), 7.21 (d, J = 8.4 Hz, 2H), 7.82 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 10.7 (CH3), 21.1 (CH), 23.5 (CH2), 39.3 (CH3), 57.6 (CH2), 128.3 (CH), 129.3 (CH), 133.6 (C), 134.6 (CH), 137.5 (C), 137.9 (C); IR (KBr/cm−1) 1672 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1489, 1279 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H17N3O4: 303.1219, found 303.1230.
C), 1489, 1279 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H17N3O4: 303.1219, found 303.1230.
1,4-Dihydro-3,5-dinitro-4-phenyl-1-propylpyridine (3Ac)
Orange solid (60.1 mg, 84%): mp 180–182 °C; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 1.08 (t, J = 7.2 Hz, 3H), 1.87 (tq, J = 7.2, 7.2 Hz, 2H), 3.58 (t, J = 7.2 Hz, 2H) 5.65 (s, 1H), 7.23–7.35 (m, 5H), 7.84 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 10.7 (CH3), 23.5 (CH2), 39.7 (CH), 57.6 (CH2), 128.1 (CH), 128.4 (CH), 128.6 (C), 133.4 (CH), 134.7 (C), 140.3 (C); IR (KBr/cm−1) 1672 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1489, 1277 (NO2). HRMS (EI, double focusing) m/z calcd. for C14H15N3O4: 289.1063, found 289.1053.
C), 1489, 1277 (NO2). HRMS (EI, double focusing) m/z calcd. for C14H15N3O4: 289.1063, found 289.1053.
4-(4-Chlorophenyl)-1,4-dihydro-3,5-dinitro-1-propylpyridine (3Ad)
Orange solid (71.3 mg, 88%): mp 143–144 °C; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 1.08 (t, J = 7.2 Hz, 3H), 1.88 (tq, J = 7.2, 7.2 Hz, 2H), 3.59 (t, J = 7.2 Hz, 2H), 5.64 (s, 1H), 7.26–7.28 (m, 4H), 7.84 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 10.7 (CH3), 23.5 (CH2), 39.3 (CH), 57.6 (CH2), 128.8 (CH), 129.7 (CH), 133.1 (C), 134.1 (CH), 134.9 (C), 138.8 (C); IR (KBr/cm−1) 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1489, 1279 (NO2). HRMS (EI, double focusing) m/z calcd. for C14H14ClN3O4: 323.0673, found 323.0670.
C), 1489, 1279 (NO2). HRMS (EI, double focusing) m/z calcd. for C14H14ClN3O4: 323.0673, found 323.0670.
1,4-Dihydro-3,5-dinitro-4-(4-nitrophenyl)-1-propylpyridine (3Ae)
Brown oil (66.9 mg, 80%); 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 1.09 (t, J = 7.2 Hz, 3H), 1.89 (tq, J = 7.2, 7.2 Hz, 2H), 3.64 (t, J = 7.2 Hz, 2H) 5.77 (s, 1H), 7.53 (d, J = 8.4 Hz, 2H), 7.90 (s, 2H), 8.17 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 10.7 (CH3), 23.5 (CH2), 39.9 (CH3), 57.8 (CH2), 123.9 (CH), 129.5 (CH), 132.5 (C), 135.5 (CH), 146.9 (C), 147.6 (C); IR (KBr/cm−1) 1681 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1506, 1278 (NO2). HRMS (EI, double focusing) m/z calcd. for C14H14N4O6: 334.0913, found 334.0905.
C), 1506, 1278 (NO2). HRMS (EI, double focusing) m/z calcd. for C14H14N4O6: 334.0913, found 334.0905.
1,4-Dihydro-3,5-dinitro-1,4-dipropylpyridine (3Af)
Brown oil (25.6 mg, 40%); 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 0.87 (t, J = 7.2 Hz, 3H), 1.00 (t, J = 7.2 Hz, 3H), 1.20 (dt, J = 4.4, 7.2 Hz, 2H), 1.64 (tq, J = 7.2, 7.2 Hz, 2H), 1.76 (tq, J = 7.2, 7.6 Hz, 2H) 3.48 (t, J = 7.6 Hz, 2H), 4.79 (t, J = 4.4 Hz, 1H), 7.77 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 10.6 (CH3), 13.9 (CH3), 18.1 (CH2), 23.5 (CH2), 33.7 (CH2), 33.9 (CH), 57.5 (CH2), 131.9 (C), 136.4 (CH); IR (KBr/cm−1) 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1506, 1277 (NO2). HRMS (EI, double focusing) m/z calcd. for C11H17N3O4: 255.1219, found 255.1213.
C), 1506, 1277 (NO2). HRMS (EI, double focusing) m/z calcd. for C11H17N3O4: 255.1219, found 255.1213.
1,4-Dihydro-3,5-dinitro-4-isobutyl-1-propylpyridine (3Ag)
Brown oil (47.1 mg, 70%); 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 0.90 (d, J = 6.4 Hz, 6H), 1.00 (t, J = 7.2 Hz, 3H), 1.46–1.49 (m, 2H), 1.78 (tq, J = 7.2, 7.2 Hz, 2H), 3.53 (t, J = 7.2 Hz, 2H), 4.78 (dt, J = 0.8, 5.2 Hz, 1H), 7.76 (d, J = 0.8 Hz, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 10.6 (CH3), 23.1 (CH3), 23.6 (CH2), 25.1 (CH), 31.8 (CH), 43.9 (CH2), 57.5 (CH2), 132.9 (C), 136.1 (CH); IR (KBr/cm−1) 1670 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1506, 1277 (NO2). HRMS (EI, double focusing) m/z calcd. for C12H19N3O4: 269.1376, found 269.1385.
C), 1506, 1277 (NO2). HRMS (EI, double focusing) m/z calcd. for C12H19N3O4: 269.1376, found 269.1385.
1,4-Dihydro-1,4-dipropyl-3-ethyl-5-nitropyridine (5Af)
Brown oil; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 0.87 (t, J = 7.2 Hz, 3H), 0.95 (t, J = 7.6 Hz, 3H), 1.07 (t, J = 7.2 Hz, 3H), 1.10–1.21 (m, 2H), 1.32–1.45 (m, 1H), 1.60–1.70 (m, 2H), 1.71–1.80 (m, 1H), 2.01–2.22 (m, 2H), 3.23–3.33 (m, 2H), 3.92 (t, J = 4.0 Hz, 1H), 5.72 (s, 1H), 7.91 (s, 1H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 10.8 (CH3), 11.6 (CH3), 14.2 (CH3), 18.0 (CH2), 23.1 (CH2), 25.2 (CH2), 32.9 (CH2), 36.3 (CH), 56.9 (CH2), 121.8 (CH), 122.9 (C), 127.8 (C), 140.1 (CH); IR (KBr/cm−1) 1670 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1456, 1219 (NO2). HRMS (EI, double focusing) m/z calcd. for C13H22N2O2: 238.1681, found 238.1689.
C), 1456, 1219 (NO2). HRMS (EI, double focusing) m/z calcd. for C13H22N2O2: 238.1681, found 238.1689.
1,4-Dihydro-4-isobutyl-3-isopropyl-5-nitro-1-propylpyridine (5Ag)
Orange oil; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 0.85 (d, J = 6.8 Hz, 3H), 0.92 (d, J = 6.8 Hz, 3H), 0.95 (t, J = 7.6 Hz, 3H), 1.07 (d, J = 6.8 Hz, 3H), 1.10 (d, J = 6.8 Hz, 3H), 1.21–1.35 (m, 1H), 1.37–1.44 (m, 1H), 1.54–1.73 (m, 3H), 2.38–2.49 (m, 1H), 3.29–3.39 (m, 2H), 3.91 (t, J = 4.4 Hz, 1H), 5.72 (s, 1H), 7.87 (s, 1H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 10.8 (CH3), 20.6 (CH3), 22.2 (CH3), 23.1 (CH3), 23.2 (CH2), 23.8 (CH3), 25.3 (CH), 30.2 (CH), 33.5 (CH), 43.9 (CH2), 57.1 (CH2), 120.9 (CH), 124.2 (C), 133.4 (C), 139.7 (CH); IR (KBr/cm−1) 1670 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1456, 1211 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H26N2O2: 266.1994, found 266.1998.
C), 1456, 1211 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H26N2O2: 266.1994, found 266.1998.
1-(tert-Butyl)-1,4-dihydro-3,5-dinitro-4-(4-methylphenyl)pyridine (3Ba)
Orange solid (58.7 mg, 74%): mp 277–278 °C; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 1.60 (s, 9H), 2.29 (s, 3H), 5.61 (s, 1H), 7.09 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 8.15 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 21.1 (CH3), 29.3 (CH3), 39.3 (CH), 60.3 (C), 128.1 (CH), 129.2 (CH), 131.5 (CH), 133.7 (C), 137.6 (C), 137.8 (C); IR (KBr/cm−1) 1668 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1497, 1269 (NO2). HRMS (ESI, TOF) m/z calcd. for C16H20N3O4 (M + H)+: 318.1454, found 318.1457.
C), 1497, 1269 (NO2). HRMS (ESI, TOF) m/z calcd. for C16H20N3O4 (M + H)+: 318.1454, found 318.1457.
1-(tert-Butyl)-1,4-dihydro-3,5-dinitro-4-(4-methoxyphenyl)pyridine (3Bb)
Orange solid (50.0 mg, 60%): mp 273–274 °C; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 1.59 (s, 9H), 3.77 (s, 3H), 5.60 (s, 1H), 6.82 (d, J = 8.4 Hz, 2H), 7.21 (d, J = 8.4 Hz, 2H), 8.15 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 29.3 (CH3), 38.9 (CH), 55.3 (CH3), 60.3 (C), 114.0 (CH), 129.4 (CH), 131.4 (CH), 132.8 (C), 133.7 (C), 159.3 (C); IR (KBr/cm−1) 1668 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1497, 1269 (NO2). HRMS (EI, double focusing) m/z calcd. for C16H19N3O5: 333.1325, found 333.1315.
C), 1497, 1269 (NO2). HRMS (EI, double focusing) m/z calcd. for C16H19N3O5: 333.1325, found 333.1315.
1-(tert-Butyl)-1,4-dihydro-3,5-dinitro-4-phenylpyridine (3Bc)
Orange solid (65.2 mg, 86%): mp 223–224 °C; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 1.60 (s, 9H), 5.65 (s, 1H), 7.23–7.30 (m, 5H), 8.17 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 29.3 (CH3), 39.7 (CH), 60.4 (C), 128.0 (CH), 128.3 (CH), 128.6 (CH), 131.7 (CH), 133.6 (C), 140.4 (C); IR (KBr/cm−1) 1670 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1487, 1271 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H17N3O4: 303.1219, found 303.1230.
C), 1487, 1271 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H17N3O4: 303.1219, found 303.1230.
1-(tert-Butyl)-4-(4-chlorophenyl)-1,4-dihydro-3,5-dinitropyridine (3Bd)
Orange solid (68.4 mg, 81%): mp 248–249 °C; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 1.60 (s, 9H), 5.63 (s, 1H), 7.23 (d, J = 8.4 Hz, 2H), 7.28 (d, J = 8.4 Hz, 2H), 8.17 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 29.3 (CH3), 39.3 (CH), 60.5 (C), 128.8 (CH), 129.6 (CH), 131.8 (CH), 133.2 (C), 134.0 (C), 138.9 (C); IR (KBr/cm−1) 1670 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1489, 1271 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H16ClN3O4: 337.0829, found 337.0825.
C), 1489, 1271 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H16ClN3O4: 337.0829, found 337.0825.
1-(tert-Butyl)-1,4-dihydro-3,5-dinitro-4-(4-nitrophenyl)pyridine (3Be)
Brown solid (65.3 mg, 75%): mp 220–221 °C; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 1.62 (s, 9H), 5.77 (s, 1H), 7.49 (d, J = 8.4 Hz, 2H), 8.17 (d, J = 8.4 Hz, 2H), 8.22 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 29.3 (CH3), 40.0 (CH), 61.0 (C), 123.9 (CH), 129.4 (CH), 132.4 (CH), 132.6 (C), 147.1 (C), 147.6 (C); IR (KBr/cm−1) 1670 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1497, 1271 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H16N4O6: 348.1070, found 348.1078.
C), 1497, 1271 (NO2). HRMS (EI, double focusing) m/z calcd. for C15H16N4O6: 348.1070, found 348.1078.
1-(tert-Butyl)-1,4-dihydro-3,5-dinitro-4-propylpyridine (3Bf)
Brown oil (17.6 mg, 26%); 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 0.88 (t, J = 4.8 Hz, 3H), 1.18 (tq, J = 4.8, 8.5 Hz, 2H), 1.51 (s, 9H), 1.65 (dt, J = 4.0, 8.5 Hz, 2H) 4.79 (t, J = 4.0 Hz, 1H), 8.10 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 13.9 (CH3), 17.9 (CH2), 29.2 (CH3), 33.6 (CH3), 33.9 (CH2), 60.0 (CH), 132.0 (C), 133.2 (C); IR (KBr/cm−1) 1670 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1489, 1260 (NO2). HRMS (EI, double focusing) m/z calcd. for C12H19N3O4: 269.1376, found 269.1384.
C), 1489, 1260 (NO2). HRMS (EI, double focusing) m/z calcd. for C12H19N3O4: 269.1376, found 269.1384.
1-(tert-Butyl)-1,4-dihydro-3,5-dinitro-4-isobutylpyridine (3Bg)
Brown oil (22.7 mg, 32%); 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 0.91 (d, J = 6.4 Hz, 6H), 1.47–1.48 (m, 3H), 1.52 (s, 9H), 4.78 (t, J = 5.2 Hz, 1H), 8.07 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 23.1 (CH3), 25.2 (CH), 29.3 (CH3), 31.8 (CH), 43.6 (CH2), 60.0 (C), 132.9 (CH), 133.1 (C); IR (KBr/cm−1) 1653 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1506, 1271 (NO2). HRMS (EI, double focusing) m/z calcd. for C13H21N3O4: 283.1532, found 283.1531.
C), 1506, 1271 (NO2). HRMS (EI, double focusing) m/z calcd. for C13H21N3O4: 283.1532, found 283.1531.
4-(4-Methoxyphenyl)-3,5-dinitropyridine (7)
Yellow solid (56.1 mg, 68%): mp 139–140 °C; 1H NMR (400 MHz, CDCl3, 30 °C, TMS) δ 3.86 (s, 3H), 6.99 (d, J = 8.8 Hz, 2H), 7.19 (d, J = 8.8 Hz, 2H), 9.15 (s, 2H); 13C NMR (100 MHz, CDCl3, 30 °C, TMS) δ 55.4 (CH3), 114.9 (CH), 119.7 (C), 128.9 (CH), 137.8 (C), 146.6 (C), 146.8 (CH), 161.4 (C); IR (KBr/cm−1) 1539 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1360, 1260 (NO2). HRMS (EI, double focusing) m/z calcd. for C12H9N3O5: 275.0542, found 275.0538.
C), 1360, 1260 (NO2). HRMS (EI, double focusing) m/z calcd. for C12H9N3O5: 275.0542, found 275.0538.
Acknowledgements
We are grateful to Mr Nobuaki Tsuda (Center for Industry, University and Government Cooperation, Nagasaki University) for the HRMS analysis.Notes and references
- (a) E. Carosati, P. Ioan, M. Micucci, F. Broccatelli, G. Cruciani, B. S. Zhorov, A. Chiarini and R. Budriesi, Curr. Med. Chem., 2012, 19, 4306–4323 CrossRef CAS; (b) R. Lavilla, J. Chem. Soc., Perkin Trans. 1, 2002, 1141–1156 RSC; (c) D. M. Stout and A. I. Meyers, Chem. Rev., 1982, 82, 223–243 CrossRef CAS.
- (a) R. Vitolina, A. Krauze, G. Duburs and A. Velena, Int. J. Pharma Sci. Res., 2012, 3, 1215–1232 CAS; (b) R. Leon and J. Marco-Contelles, Curr. Med. Chem., 2011, 18, 552–576 CrossRef CAS; (c) N. Edraki, A. R. Mehdipour, M. Khoshneviszadeh and R. Miri, Drug Discovery Today, 2009, 14, 1058–1066 CrossRef CAS PubMed; (d) K. Singh, D. Arora, K. Singh and S. Singh, Mini-Rev. Med. Chem., 2009, 9, 95–106 CrossRef CAS; (e) D. J. Trigglen, Drug Dev. Res., 2003, 58, 5–17 CrossRef PubMed.
- (a) G. Swarnalatha, G. Prasanthi, N. Sirisha and C. C. Madhusudhana, Int. J. ChemTech Res., 2011, 3, 75–89 CAS; (b) R. Miri and A. Mehdipour, Bioorg. Med. Chem., 2008, 16, 8329–8334 CrossRef CAS PubMed; (c) X. Chen, H. Li, C. W. Yap, C. Y. Ung, L. Jiang, Z. W. Cao, Y. X. Li and Y. Z. Chen, Cardiovasc. Hematol. Agents Med. Chem., 2007, 5, 11–19 CrossRef CAS.
- (a) C. Coburger, J. Wollmann, C. Baumert, M. Krug, J. Molnar, H. Lage and A. Hilgeroth, J. Med. Chem., 2008, 51, 5871–5874 CrossRef CAS PubMed; (b) M. Richter, J. Molnar and A. Hilgeroth, J. Med. Chem., 2006, 49, 2838–2840 CrossRef CAS PubMed; (c) A. Hilgeroth, A. Billich and H. Lilie, Eur. J. Med. Chem., 2001, 36, 367–374 CrossRef CAS; (d) A. Hilgeroth, U. Baumeister and F. W. Heinemann, Eur. J. Org. Chem., 2000, 245–249 CrossRef CAS.
- Recently, Sueki and Shimizu et al. reported synthesis of 2,6-unsubstituted 1,4-DHPs and their photoelectronic properties: S. Sueki, R. Takei, Y. Zaitsu, J. Abe, A. Fukuda, K. Seto, Y. Furukawa and I. Shimizu, Eur. J. Org. Chem., 2014, 5281–5301 CrossRef CAS PubMed and references are therein.
- (a) A. Hantzsch, Chem. Ber., 1881, 14, 1637–1638 CrossRef PubMed; (b) A. Hantzsch, Justus Liebigs Ann. Chem., 1882, 215, 1–82 CrossRef PubMed.
- Y. Nakaike, N. Nishiwaki, M. Ariga and Y. Tobe, J. Org. Chem., 2014, 79, 2163–2169 CrossRef CAS PubMed.
- Rao et al. reported the synthesis of 2,4,6-substituted-3,5-dinitro-DHPs by pseudo three-component reaction of nitroketene-N,S-acetals and aldehydes, which is relevant to the present study: H. S. P. Rao and A. Parthiban, Org. Biomol. Chem., 2014, 12, 6223–6238 Search PubMed.
- Recent example for using 3,5-dinitropyridine as synthetic intermediate: S. Lee, S. Diab, P. Queval, M. Sebban, I. Chataigner and S. R. Piettre, Chem.–Eur. J., 2013, 19, 7181–7192 CrossRef CAS PubMed.
- (a) J. Šturala, S. Boháčová, J. Chudoba, R. Metelková and R. Cibulka, J. Org. Chem., 2015, 80, 2676–2699 CrossRef PubMed; (b) M. Winkler, B. Cakir and W. Sander, J. Am. Chem. Soc., 2004, 126, 6135–6149 CrossRef CAS PubMed; (c) E. Plazek, Recl. Trav. Chim. Pays-Bas, 1953, 72, 569–575 CrossRef CAS PubMed.
- (a) S. G. A. Moinuddin, B. Youn, D. L. Bedgar, M. A. Costa, G. L. Helms, C. Kang, L. B. Davin and N. G. Lewis, Org. Biomol. Chem., 2006, 4, 808–816 RSC; (b) S. Nakano, W. Sakane, H. Oinaka and Y. Fujimoto, Bioorg. Med. Chem., 2006, 14, 6404–6408 CrossRef CAS PubMed; (c) C. R. Pudney, S. Hay, M. J. Sutcliffe and N. S. Scrutton, J. Am. Chem. Soc., 2006, 128, 14053–14058 CrossRef CAS PubMed.
- (a) Y. Lu, F. Qu, B. Moore, D. Endicott and W. Kuester, J. Org. Chem., 2008, 73, 4763–4770 CrossRef CAS PubMed; (b) Y. Lu, D. Endicott and W. Kuester, Tetrahedron Lett., 2007, 48, 6356–6359 CrossRef CAS PubMed.
- G. P. Sagitullina, L. V. Glizdinskaya and R. S. Sagitullin, Chem. Heterocycl. Compd., 2005, 41, 739–744 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra for new compounds, the details about optimization of reaction conditions, and X-ray crystallographic data (CIF file), ORTEP drawing for 3ab. CCDC 1416840. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra19439c |
| ‡ Present address: Institute for Chemical Research, Kyoto University Uji, Kyoto 611-0011, Japan. |
| This journal is © The Royal Society of Chemistry 2015 |