Greatly enhanced H2S sensitivity using defect-rich titanium oxide films†
Tushar C. Jagadale*,
Vidya Prasad,
Niranjan S. Ramgir,
Champalal Prajapat,
Uday V. Patil,
Anil Debnath,
D. K. Aswal and
S. K. Gupta
Technical Physics Division, Bhabha Atomic Research Centre, Trombay, Mumbai – 400 085, India. E-mail: jagadale@barc.gov.in
First published on 22nd October 2015
Abstract
Defect-rich titanium oxide films were prepared using the laser ablation technique. The target pellet used for the ablation was rutile phase pure stoichiometric TiO2. X-ray photoelectron and synchrotron soft X-ray absorption spectroscopy measurements have revealed the chemical composition of the films. Using 200 mJ of laser energy, the stoichiometric transfer of TiO2 from the target to the substrate was achieved. The films prepared using 500 mJ of laser energy were observed to be highly defect-rich with a composition of Ti/TiOx. The gas sensing characteristics of these films were tested. The unique defect-richness of the films favours the formation of excessive overlayers of chemisorbed oxygen on the surface of the films in large proportions as compared to the lattice oxygen, making the films rich in titanium forming Ti–Ti electronic bonds, producing a great enhancement in the sensitivity toward highly toxic H2S gas.
Introduction
H2S is one of the common gases produced in various industrial processes, which is highly toxic and corrosive in nature. It is a colourless, low-lying and extremely flammable gas. Its leakage poses potential danger to the working environment. Humans exposed to the gas suffer eye irritation, olfactory fatigue, and damage to the lungs and nervous system. The threshold exposure limit value for H2S is 10 ppm. Inhalation of the gas at concentrations as low as 320 ppm may collapse the heartbeat leading to sudden death1–3 Thus, the critical monitoring and controlling of H2S is crucial from a safety point of view in many laboratories and industrial areas. Moreover, the real time detection of H2S gas produced by mammalian cells is also a hot topic of research due to the proven role of H2S as a signaling molecule for many physiological functions and because its abnormal generation causes human diseases.4,5 Recently there have been reports on oxide based H2S sensors such as CuO,6 ZnO,7 WO3,8 SnO2 9 etc. but very few are suitable for use in sensor technology unless they are modified or doped by another material. Specifically, tin oxide is sensitive to many gases raising the issue of selectivity.10 Thus, developing new materials with a better selectivity and higher response is extremely important. So far, although research exploring H2S sensors using TiO2 (ref. 11 and 12) is relatively new, nevertheless some efforts have been made which are as detailed below.E. D. Gaspera et al.13 reported Au-NPs dispersed in TiO2–NiO composite films for H2S sensing at an operating temperature of 400 °C with a very poor response. Curry and co-workers14 fabricated a single TiO2 nanowire device and explored this for H2S sensing between 10–80 ppm, which seems less significant for large scale industrial use. Chaudhari et al.15 proposed TiO2/Al2O3/Pd and TiO2/ZnO/CdO composite electrodes for H2S sensing with a poor response at an operating temperature of about 300 °C. Topalian et al.16 detected low concentrations of H2S using noise spectroscopy when a TiO2 gas sensor was irradiated with UV light with a mere response value of ∼10. In their report, no response was observed in the absence of UV. H2S detection by Pt-doped TiO2 NC based gas sensors showed the highest sensitivity of about 70, which is quite low compared to our values of towards 250 ppm H2S/air at 500 °C by 4.3 at% Pt-doped sensors at an operating temperature of 500 °C.17 Hydrothermally synthesized rice-grain shaped TiO2 nanostructures are used for thick film preparation using the screen printing technique and when tested for H2S gas showed a sensitivity (SR%) of around 100 at 1000 ppm.18 Upon a critical review of the literature in the context of titanium oxide films and nanosystems as H2S sensors, none of the reports were observed to be exclusively on undoped titanium oxide films or to explore the promising capability for the realization of commercial sensor technology for highly toxic H2S with an extra-ordinary response value.
TiO2 is really a very promising material due to its anti-corrosive and chemically stable nature, with its band gap tailoring ability leading to electronic structure variations. It is mainly studied in the context of dye sensitized solar cells19 and photo-catalysis.20 Recently improved surface reactivity in TiO2 systems has been reported where ultrathin nanotubes,21 nanohybrids22 and sensitizers23 have been used. The band gap of titanium oxide is engineered by doping with metal/non-metal24 elements that alter the electronic structure and enhance the surface-activity of TiO2. Although the role of bulk and surface defects in oxides has been studied in the context of magnetism,25 such defect chemistry can play an important role in surface-sensitive phenomena such as sensing and catalysis. Thereby, it is worth investigating the engineering of surface defects in undoped titanium oxide films as it can lead to newer functionalities.
Herein, we report explicitly prepared defect-rich undoped titanium oxide films using the laser ablation technique for gas sensing applications using a set-up developed in our laboratory. Since sensing is a surface activated phenomenon, special emphasis has been given to engineering the surface defects of the films under identical preparative parameters except for a variation in the laser energy used for ablation. The objective of the present study is to form defect-rich films by varying the laser ablation energy for the sensing of highly toxic H2S.
Experimental
In a typical experiment, the TiO2 target was pelletized using the spark plasma sintering technique and was used for the laser ablation. The SEM and EDS measurements of the TiO2 pellet were performed using a TESCAN, Model: TS 5130 MM, Oxford Instruments. A KrF laser source (make: Lambda Physik) with a wavelength of 248 nm was used to ablate the TiO2 pellet on a (001) LaAlO3 (LAO) substrate at 600 °C under an oxygen partial pressure of 1 × 10−5 Torr for 10 min. The LAO substrates were chemically cleaned before the deposition. The laser pulse frequency and energy density at the target surface were kept at 10 Hz and 2 J cm−2 respectively for the deposition. After the deposition, the substrates were cooled down to room temperature under the same oxygen partial pressure as was used during the deposition. The films were prepared using three different laser energies 200, 500 and 680 mJ. These films were characterized with X-ray photo-electron spectroscopy, using an Mg Kα source (make: RIBER system), and X-ray absorption spectroscopy (Synchrotron, INDUS-2 at Indore, India) to reveal the surface defects of the films and were further investigated for their H2S sensing ability using a laboratory made set-up. The H2S gas sensing measurements were performed on the films using the actual gas sensing set-up as shown in ESI-I† along-with its schematic shown on the right. In brief, the electrode contacts were defined by a 120 nm thick Au layer (thermal evaporation) with 1 mm spacing. The sensor temperature was controlled using a controller circuit and two Pt-100s attached at the backside of the substrate (heater). The response curve (current as a function of time) was recorded upon exposure to different concentrations of H2S using a personal computer (PC) equipped with Labview software. The required concentration of the test gas in the chamber was achieved by injecting a known amount of the gas using a syringe. The sensor response was calculated from the response curves using the formula:| SR% = (Ig − Ia)/Ia × 100% | (i) |
The response and recovery times of the films were calculated from the response curves. The response time was taken as the time required to reach 90% of the total change in the current upon exposure to the test gas. The recovery time was taken as the time required to return to 10% of the original baseline signal upon removal of the test gas.
Results and discussion
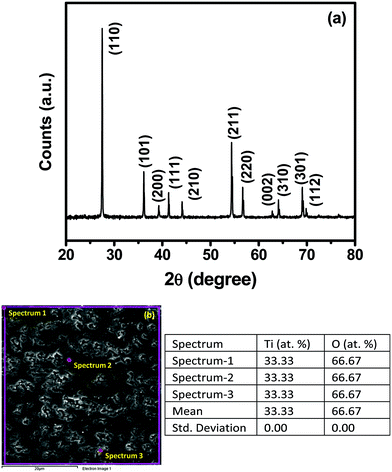
Fig. 1(a) shows the XRD data from the TiO2 pellet used as a target for pulsed laser ablation. The pellet is sintered by the spark plasma technique. The pellet is crystallized in the pure rutile phase. Fig. 1(b) shows the SEM image and EDS measurement data for the pellet. It shows the stoichiometric TiO2 composition of the pellet without defects. The table in Fig. 1(b) shows the Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio is 1
O ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. This defect-free and stoichiometric TiO2 pellet is used to prepare defect-rich non-stoichiometric, as well as defect-free stoichiometric, films of titanium oxide at different laser energies using the pulsed laser technique.
2. This defect-free and stoichiometric TiO2 pellet is used to prepare defect-rich non-stoichiometric, as well as defect-free stoichiometric, films of titanium oxide at different laser energies using the pulsed laser technique.
Defect-rich titanium oxide thin films were prepared using critically optimised 500 mJ laser ablation energy and were tested for H2S sensing and the resulting response kinetics are shown in Fig. 2(a). They exhibit an extra-ordinary sensor response equal to 102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 867% at a 50 ppm H2S concentration which is conceivably the best response value for titania in the literature. It is remarkable to note the great enhancement in the sensing response of the titanium oxide film processed using 500 mJ laser energy. The response increases in a cumulative manner with the successive addition of 5 ppm of H2S gas into the measurement chamber in regular steps. Additionally, the sensor film exhibited a highly selective response towards H2S with a negligible or almost zero response towards other gases.
867% at a 50 ppm H2S concentration which is conceivably the best response value for titania in the literature. It is remarkable to note the great enhancement in the sensing response of the titanium oxide film processed using 500 mJ laser energy. The response increases in a cumulative manner with the successive addition of 5 ppm of H2S gas into the measurement chamber in regular steps. Additionally, the sensor film exhibited a highly selective response towards H2S with a negligible or almost zero response towards other gases.
Fig. 2(b) shows the variation of the sensor response with respect to different gases which confirmed that the films are exclusively sensitive to H2S with almost no response to the other gases. The initial increase in the sensor response with respect to the gas concentration and its eventual equilibrium are shown in Fig. 2(c). The black curve for the sample prepared at 500 mJ laser energy fits well with eqn (ii), given as:
| y = a + bxc | (ii) |
A moderate sensing response (SR%) of ∼1000 was observed for titanium oxide films prepared using 680 mJ laser energy as shown in Fig. 2(c). This investigation was done to check the effect of a further increase in laser energy on the H2S response. The thicknesses of the films prepared using different laser energies are in the range of 55–100 nm (measured using an ellipsometry technique). However, gas sensing is purely a surface active phenomenon wherein the bulk contribution is often negligible. Thus, it is imperative to infer that thickness has no significant role to play in the H2S sensing response.27,28
A prominent fact to note from Fig. 3(A) is the initial increase in the current of the film which after exposure to a 5 ppm H2S concentration tends towards near saturation unlike the film prepared using 500 mJ laser energy wherein the saturation stage started at a later concentration. This implies that there is a significant difference in the defective surface structure of the film prepared using 680 mJ as compared to the film prepared using 500 mJ laser energy. Thus, the defect-rich unique composition of the film prepared using 500 mJ laser energy is solely responsible for the great enhancement in H2S sensitivity. Though rich in defects, the exactly similar composition of film has not been achieved in the case of using 680 mJ laser energy.
A defect-free stoichiometric TiO2 film was prepared using critically optimised 200 mJ laser energy and was tested for H2S sensing. The data is shown in Fig. 3(B). It is observed that the sensing response increases with an increase in the H2S gas concentration but the sensor response (SR%) is of the order of ∼100 even at a 50 ppm gas concentration. These films showed response and recovery times of 150 s and 2500 s respectively for 10 ppm of H2S. Fig. 3(C) shows the sensor response of TiO2 thin films prepared using laser energy of 200 mJ at different temperatures with varying gas concentrations. It indicates that the maximum sensor response is achieved at 100 °C which confirms that the optimum sensor operating temperature is 100 °C which is relatively low. Fig. 3(D) supports the same with a similar trend in the SR% observed for different laser energies.
It is very important to note the faster and greatly enhanced response kinetics towards H2S by the defect-rich titanium oxide films prepared using 500 mJ laser energy as compared to the defect-free TiO2 films made using 200 mJ laser energy. Another prominent feature of the film sensor was its extremely quick (<5 min) recovery after turning off the H2S, showing switch-like behaviour. A relatively shorter response time was also noted. Interestingly, we further pursued sub-ppm level H2S-sensing that showed a better response as shown in the inset of Fig. 2(a). Since the film was prepared at 600 °C, it is very stable. This can certainly meet three requirements, sensitivity, selectivity and stability, for the realization of H2S sensor technology.
Fig. 4 summarizes the XPS results for the titanium oxide films prepared using 200 mJ and 500 mJ laser energies. The Ti 2p3/2 & 2p1/2 core levels for titanium oxide prepared using laser energy of 200 mJ were seen to appear at 458.3 eV and 464 eV, respectively with a peak separation of 5.7 eV. Also, both Ti core-levels are symmetric in nature ruling out the possibility of reduced species like Ti2+ or Ti3+. This proves the formation of stoichiometric titanium oxide. However for the films prepared at 500 mJ and 680 mJ, the Ti 2p3/2 core levels showed a considerable shift to higher binding energies, to 461.5 eV and 460.8 eV, and also a similar outcome in the case of the 2p1/2 level, to 467.4 eV and 466.5 eV, was noted. Please see ESI (III)† for the XPS spectra of films prepared using 680 mJ laser energy. The difference in energies of the 2p3/2 and 2p1/2 core-levels is almost the same in all cases, i.e. 5.7 eV. This shows the formation of oxidized titanium. The slight shift in peaks towards a lower BE was observed in the case of the 680 mJ film as compared to the 500 mJ film indicating more stoichiometric TiOx formation. Additionally a small contribution can be noted around 453 eV and 460 eV in the data for the 500 mJ & 680 mJ samples. This can be attributed to the presence of 2p3/2 and 2p1/2 core-levels for Ti0 i.e. metallic titanium in trace amounts. This invites discussion.
 | ||
| Fig. 4 XPS spectra (Ti 2p, O 1s) of titanium oxide films prepared using (a) 200 mJ, (upper panels) and (b) 500 mJ, (lower panels) laser energy. | ||
A literature survey shows very few reports on the XPS results of such types of uncommon titanium oxide systems. The most relevant reference was reported in 1987 by Ocal & co-workers29 wherein they characterized with XPS thin films of titanium oxide grown by oxidizing a Ti (0001) surface. They studied the evolution of the Ti 2p XPS spectrum during the oxidation of a Ti surface and the consequent thermal treatment. At plus 5 mTorr of O2 at 400 K, a completely oxidized surface was obtained. Basically they stated that the oxide film had grown since the electronic equilibrium among the gas–oxide and metal–oxide interfaces established an electric field in the oxide that had driven oxygen anions through it from the gas–oxide to the metal–oxide interface. The basic idea was to realize that oxygen anions may diffuse across the oxide since its electron affinity energy is larger than the work function of the Ti-metal. Subsequently the existence of anionic overlayers that were stable at or below room temperature was also reported. Cut short, this report showed that the formation of the Ti/TiOx composition of the grown film had excessive oxygen over-layers as evidenced by the XPS results and those data are matching exactly with our XPS results. Another recent report on the existence of Ti–Ti electronic bonding gave quite similar XPS findings with enhanced catalytic activity.30
Quantitative estimates of the ratio of Olattice/Ochemi-sorbed species were also calculated from the XPS data (see Table 1). In the case of the films made using 500 mJ, this ratio comes out to be nearly 540% whereas for 200 mJ it is just 67% and for the 680 mJ samples it is considerable, i.e. ∼330%, reflecting clearly the H2S sensitivity. So, the main contributing factor towards the enhancement in H2S sensitivity is the excessive chemically adsorbed oxygen on the defect-rich films made using 500 mJ laser energy. Such a defect-rich off-stoichiometric titanium oxide film has a better sensitivity than a stoichiometric film.
| Defect-free TiO2 film prepared using 200 mJ laser energy | ||
|---|---|---|
| Area under curve | Relative proportion in the film | |
| Metallic Ti | — | — |
| Oxidised Ti | 2P1/2: 2127, 2P3/2: 4288 | |
| Olattice | O 1s: 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952 952 |
60% |
| Oadsorbed | O 1s: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162 162 |
40% |
| Defect-rich titanium oxide film prepared using 500 mJ laser energy | ||
|---|---|---|
| Area under curve | Relative proportion in the film | |
| Metallic Ti | 2P1/2: 1450, 2P1/2: 2888 | |
| Oxidised Ti | 2P1/2: 5626, 2P1/2: 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135 135 |
|
| Olattice | O 1s: 3010 | 10% |
| Oadosrbed | O 1s: 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 979, O 1s: 9125 (–OH) 979, O 1s: 9125 (–OH) |
Ochemi-sorbed: 54% and 36% oxygen bonded with carbon and –OH |
| Defect-rich titanium oxide film prepared using 680 mJ laser energy | ||
|---|---|---|
| Area under curve | Relative proportion in the film | |
| Metallic Ti | 2P1/2: 2008, 2P1/2: 4000 | |
| Oxidised Ti | 2P1/2: 7684, 2P1/2: 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668 668 |
|
| Olattice | O 1s: 3174 | 10% |
| Oadsorbed | O 1s: 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728, O 1s: 19 728, O 1s: 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 (–OH) 743 (–OH) |
Ochemisorbed: 33% and 57% oxygen bonded with carbon and –OH |
To confirm the XPS findings, these films were further characterized using X-ray absorption spectroscopy (XAS) to understand the surface electronic structure. The soft X-ray absorption near edge structure (XANES) experiment was carried out using an Indus-2 beamline-1 (BL-1) at the RRCAT, Indore, India. The spectra at the Ti L-edge (∼458 eV) were acquired at room temperature using the TEY method. The XANES spectra were processed to obtain normalized absorbance. Further, the spectra have been analyzed using the “fingerprint” method by comparing a sample’s spectra with those taken for reference bulk compounds. The complete data is shown in ESI IV.†
The Ti L3,2-edge spectra as shown in Fig. 5a demonstrate four different features at 458.1 eV, 460.7 eV, 463.5 eV and 465.8 eV, namely A, B (B1; B2), C, and D, respectively. These four main peaks are the L3 and L2 absorption peaks wherein each peak splits up into doublets i.e. into eg and t2g (L3: 457–462 eV and L2: 462–468 eV) due to the crystal field and spin–orbit interactions of the Ti-core levels.31 The features of the L3- and L2-edge are related to the transitions from the 2p3/2 to 3d5/2 and 2p1/2 to 3d3/2 states respectively. The absorption edge of all the samples is almost same i.e. around 458 eV indicating a maximum of Ti species in a single oxidation state, i.e. Ti4+, that corroborates with the XPS findings where the absence of Ti2+/Ti3+ species was observed. Nonetheless, Ti metallic species show similar features without splitting.32 Its clear indication is not visible in the spectra for the films prepared using 500 and 680 mJ laser energy due to overlapping features with oxidised titanium and the relatively lower proportion of Ti0 in the films. Since the egB1 feature is more related to a defect-rich phase, its pronounced appearance in the film prepared using 500 mJ laser energy supports the defect-richness in the film.32
 | ||
| Fig. 5 XANES spectra of titanium oxide films at the (a) Ti L3,2-edge and (b) O K-edge for films made using energy of 200 mJ and 500 mJ. Data for 680 mJ energy with a bulk reference are shown in ESI-VI.† | ||
Fig. 5b presents the features of the O K-edge XANES spectra of the samples. There are four major peaks present in the O K-edge spectra of the films at 530.7 eV, 532.8 eV, 539.8 eV and 543.1 eV. These features can be distinguished in two different energy ranges viz. first below 535 eV owing to transitions from the O 1s to unoccupied 2p states and second above 535 eV attributed to the existence of complex wide bands relating to transitions to the antibonding O 2p and Ti 4sp states. The presence of some extra black arrowed absorption peaks indicates the existence of chemisorbed oxygen overlayers on the films.32 The broadening of the peaks on the higher energy side is not only due to disorder but it also reflects important surface chemical changes.33 The appearance of such interesting features is intriguing and requires further investigations that are underway, using techniques like synchrotron EXAFS, SPM and DFT calculations to reveal the detailed surface electronic structure of defect rich titanium oxide films. Thus, the XPS and XANES investigation reveals overlayers of chemisorbed oxygen in the case of defect rich titanium oxide films.
Further, Fig. 6(a) shows the response curves recorded upon repetitive exposure to 2 and 3 ppm H2S. The sensor films exhibited similar response values with identical response kinetics indicating their high repeatability. Moreover, the long term stability measurements as shown in Fig. 6(b) indicated that the sensor’s response is highly stable over the measurement period of 30 days. No significant variations in the response values were observed indicating the highly stable behaviour of the film based sensors.
Furthermore, the higher electrical resistivity of the defect-rich films as compared to the defect-free films was observed from the H2S sensing data taken using the cumulative method as shown in Fig. 2(a) and 3(B). This is due to the excessive chemisorbed oxygen preventing inter-grain charge transport. Titanium oxide films prepared using 200 mJ energy showed an initial current of about 10 μA and films prepared using 500 mJ energy showed an initial current of about 10 nA at the same temperature revealing that films prepared using 500 mJ energy were highly resistive in nature. This increase in resistivity is attributed to the excessive overlayers of chemisorbed oxygen on the film surface.
These chemisorbed oxygen species play a crucial role in enhancing the H2S gas sensitivity as explained by the schematic diagram in Fig. 7. These chemically adsorbed oxygen species, due to a higher oxidation potential, accept electrons from the oxide surface thereby creating electron-deficient layers. The H2S reacts with these oxygen species and gets dissociated after releasing trapped electrons via either of the speculated reactions given below:
| 2H2S(g) + 2O2−(ads) → 2H2(g) + 2SO2(g) + 3e− | (iii-a) |
| 2H2S(g) + 3O2−(ads) → 2H2O(g) + 2SO2(g) + 3e− | (iii-b) |
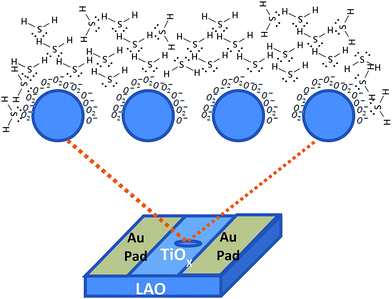 | ||
| Fig. 7 Schematic diagram demonstrating the H2S sensing mechanism by defect-rich titanium oxide film. | ||
This release of large number of trapped electrons decreases the thickness of the electron depletion layer that facilitates access for charge carriers into the conduction band of the film which was evident as a sharp increase in the conductivity of the film. The process was reversed if H2S was removed and the film was exposed to ambient conditions. In other words, the adsorbed oxygen on the surface causes the oxidation of H2S and during recovery the adsorption of ambient oxygen species easily refreshes the sensor surface.
Thereby herein, the interaction with the H2S gas is primarily governed by the nature of the adsorbed oxygen species. The surface active defects facilitate the chemical adsorption of oxygen which oxidizes the target gas. The exposed H2S gets dissociated on donating electrons to the sensor via chemisorbed oxygen, thereby lowering the resistance; thus such types of sensors are often categorized as chemi-resistive sensors. The unique strength of our defect-rich titanium oxide films is the availability of enormous amounts of surface states that facilitate the chemical adsorption of ambient oxygen in significant amounts which greatly enhances the H2S sensitivity.
Photographs of the LAO substrate and the defect-rich TiOx film, with an attempt to show its optical transparency, are shown in Fig. 8(a) and (b). The actual sensor film mounted on a Teflon head and the temperature control circuit and the SS housing used for sensor packaging are shown in Fig. 8(c) and (d). This clearly demonstrates the high potential of defect-rich TiOx films towards the realization of H2S sensor technology.
Ultimately it is necessary to project the possible causes of the formation of highly defect-rich Ti/TiOx film using 500 mJ laser energy over the relatively less defective and pure TiO2 film formation at laser energies of 680 mJ and 200 mJ. PLD is popular for the stoichiometric transfer of material from target to substrate. Nevertheless, it is well-known that such favourable results do not occur under all experimental conditions. By controlling the experimental parameters like the gas pressure, laser energy, etc. the target material composition can be harmoniously transferred to the growing film. In the cases of all the films under investigation, the growing conditions were the same except for the laser energy. So, it is quite straightforward that the changes in the stoichiometry of the films occurred in the plume rather than at the target or substrate. It is further inferred that the non-harmonious transfer is due to differential scattering in the plume itself. This can be due to differences in the mass of the species or differences in the charge states of the species, or due to the rapid expansion of ionic species in the plume under or near to vacuum (∼10−5 Torr) conditions. The time lag between the ablation of the target materials and particulate formation on the substrate is the crucial factor. A higher time lag means a slower movement of species, leading to negligible scattering events.34 Thus, 200 mJ of laser energy yields a lower ablation rate, and a slower transfer to the substrate is optimised to form stoichiometric TiO2 however in the latter cases, higher laser energy yields a higher ablation rate, thus species did not have enough time to combine successfully due to differences in their masses and charge states, encountering many scattering events thereby getting disparately accumulated on substrates. Thus, defect-rich TiOx film formation is attributed to the non-harmonious transfer of the ablated species leading to differential scattering in the plume. Moreover, such defect-rich films are perfectly reproducible so PLD has also the potential for the non-congruent transfer of materials to a substrate with greater accuracy and reproducibility which finds extreme usability for certain surface activated applications such as sensors or catalysis.
Conclusions
In conclusion, we have successfully demonstrated the explicit preparation of defect-rich titanium oxide films of the composition Ti/TiOx exhibiting an extra-ordinary H2S response (SR% > 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). This remarkable and exclusive sensing response towards H2S is conceivably the best in the literature. Moreover, this unique film composition was highly reproducible by pulsed laser deposition. The enhanced response characteristic is attributed to the enormous surface defects arising from the lattice oxygen-deficiency and Ti–Ti electronic bonding that facilitate the formation of over-layers of chemisorbed oxygen species. Further these films exhibited quicker recovery and better stability thereby emphasising their suitability for the realization of sensor technology. In addition, these defect rich films may find usefulness in visible light catalysis or solar cells due to their Ti–Ti electronic interactions that help to narrow the band gap.
000). This remarkable and exclusive sensing response towards H2S is conceivably the best in the literature. Moreover, this unique film composition was highly reproducible by pulsed laser deposition. The enhanced response characteristic is attributed to the enormous surface defects arising from the lattice oxygen-deficiency and Ti–Ti electronic bonding that facilitate the formation of over-layers of chemisorbed oxygen species. Further these films exhibited quicker recovery and better stability thereby emphasising their suitability for the realization of sensor technology. In addition, these defect rich films may find usefulness in visible light catalysis or solar cells due to their Ti–Ti electronic interactions that help to narrow the band gap.
Acknowledgements
One of the authors, TJ, acknowledges DST, Govt. of India for the INSPIRE Faculty Award. Thanks are due to D. K. Shukla and D. M. Phase (UGC-DAE CSR, Indore) for providing XAS measurements. Authors also acknowledge Dr V. Saxena for her help with thickness measurements. Prof. D. C. Kothari, Mumbai University is also thanked for his valuable guidance and support.Notes and references
- D. K. Aswal and S. K. Gupta, Science and technology of chemi-resisitive sensors, Nova Publishers, 2007 Search PubMed.
- M. Lancia, L. Panata, V. Tondi, L. Carlini, M. Bacci and R. Rossi, Am. J. Forensic Med. Pathol., 2013, 34, 315–317 CrossRef PubMed.
- OSHA 3300–2005, document of the Occupational Safety & Health Administration, Department of Labor, United States, Link: http://www.OSHA.gov/OshDoc/data_Hurricane_Facts/hydrogen_sulfide_fact.pdf.
- C. Zhao, X. Zhang, K. Li, S. Zhu, Z. Guo, L. Zhang, F. Wang, Q. Fei, S. Luo, P. Shi, H. Tian and W. Zhu, J. Am. Chem. Soc., 2015, 137, 8490–8498 CrossRef CAS PubMed.
- L. Wei, Z. Zhu, Y. Li, L. Yi and Z. Xi, Chem. Commun., 2015, 51, 10463–10466 RSC.
- Y. Qin, F. Zhang, Y. Chen, Y. Zhou, J. Li, A. Zhu, Y. Luo, Y. Tian and J. Yang, J. Phys. Chem. C, 2012, 116, 11994–12000 CAS.
- J. Kim and K. Yong, J. Phys. Chem. C, 2011, 115, 7218–7224 CAS.
- W. Mickelson, A. Sussman and A. Zettl, Appl. Phys. Lett., 2012, 100, 173110–173114 CrossRef.
- S. J. Choi, B. H. Jang, S. J. Lee, B. K. Min, A. Rothschild and I. D. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 2588–2597 CAS.
- S. Das and V. Jayraman, Prog. Mater. Sci., 2014, 66, 112–255 CrossRef CAS.
- X. Zhou, S. Lee, Z. Xu and J. Yoon, Chem. Rev., 2015, 115, 7944–8000 CrossRef CAS PubMed.
- J. Bai and B. Zhou, Chem. Rev., 2014, 114, 10131–10176 CrossRef CAS PubMed.
- E. D. Gaspera, M. Guglielmi, S. Agnoli, G. Granozzi, M. L. Post, V. Bello, G. Mattei and A. Martucci, Chem. Mater., 2010, 22, 3407–3417 CrossRef.
- M. Munz, M. T. Langridge, K. K. Devarepally, D. C. Cox, P. Patel, N. A. Martin, G. Vargha, V. Stolojan, S. White and R. J. Curry, ACS Appl. Mater. Interfaces, 2013, 5, 1197–1205 CAS.
- G. N. Chaudhari, D. R. Bambole, A. B. Bodade and P. R. Padole, J. Mater. Sci., 2006, 41, 4860–4864 CrossRef CAS.
- Z. Topalian, J. M. Smulko, G. A. Niklasson and C. G. Granqvist, J. Phys.: Conf. Ser., 2007, 76, 012056 CrossRef.
- H. Lin, T. Hsu, C. Tung and C. Hsu, Nanostruct. Mater., 1995, 6(5), 1001–1004 CrossRef.
- G. J. Mogal, D. V. Ahire, G. E. Patil, F. I. Ezema and G. H. Jain, Chem. Sci. Trans., 2015, 4(1), 296–302 CAS.
- B. O’regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- A. M. Mohamed, A. S. Aljaber, S. Y. AlQaradawiand and N. K. Allam, Chem. Commun., 2015, 51, 12617–12620 RSC.
- W. Tu, Y. Zhou, S. Feng, Q. Xu, P. Li, X. Wang, M. Xiaoand and Z. Zou, Chem. Commun., 2015, 51, 13354–13357 Search PubMed.
- K. Li, L. Lin, T. Peng, Y. Guo, R. Li and J. Zhang, Chem. Commun., 2015, 51, 12443–12446 RSC.
- T. C. Jagadale, S. P. Takale, R. S. Sonawane, H. M. Joshi, S. I. Patil, B. B. Kale and S. B. Ogale, J. Phys. Chem. C, 2008, 112, 14595–14602 CAS.
- S. B. Ogale, Adv. Mater., 2010, 22, 3125–3155 CrossRef CAS PubMed.
- A. K. Debnath, S. Samanta, A. Singh, D. K. Aswal, S. K. Gupta and J. V. Yakhmi, Chem. Phys. Lett., 2009, 480, 185–188 CrossRef CAS.
- N. S. Ramgir, N. Datta, M. Kaur, S. Kailasaganapathi, A. K. Debnath, D. K. Aswal and S. K. Gupta, Colloids Surf., A, 2013, 439, 101–116 CrossRef CAS.
- N. S. Ramgir, Y. Yang and M. Zacharias, Small, 2012, 6, 1705–1722 CrossRef PubMed.
- C. Ocal and S. Ferrer, Surf. Sci., 1987, 191, 147–156 CrossRef CAS.
- H. Tong, N. Umwzawa and J. Ye, Chem. Commun., 2011, 47, 4219–4221 RSC.
- L. Soriano, M. Abbate, J. Vogel, J. C. Fuggle, A. Fernandez and A. R. Gonz Blez-Elipe, Surf. Sci., 1993, 290, 427–435 CrossRef CAS.
- A. A. Mosquera, J. L. Endrino and J. M. Albella, J. Anal. At. Spectrom., 2014, 29, 736–742 RSC.
- M. Fusi, E. Maccallini, T. Caruso, C. S. Casari, A. Li Bassi, C. E. Bottani, P. Rudolf, K. C. Prince and R. G. Agostino, Surf. Sci., 2011, 605, 333–340 CrossRef CAS.
- C. B. Arnold and M. J. Aziz, Appl. Phys. A, 1999, 69, S23–S27 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19426a |
| This journal is © The Royal Society of Chemistry 2015 |